Chemical Physics ( IF 2.3 ) Pub Date : 2020-11-28 , DOI: 10.1016/j.chemphys.2020.111060 Yanbiao Wang , Tingting Zhang , Liyan Zhu , Xu Wang , Qijun Shao
|
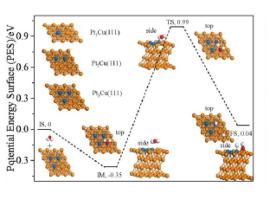
Transition metal alloys have been proposed as an effective strategy to achieve effective and selective heterogeneous catalysts for the industrially important hydrogen production reaction. First-principles calculations have been performed to explore catalytic activity of PtnCu(111) (n = 1−3) surface alloys, in which the Pt monomer, Pt dimer, or Pt trimer is incorporated into the surface layer of Cu(111), for the water-gas shift reaction (WGSR). Our results reveal that the Pt monomer-doped Cu(111) surface show much lower barrier for the H migration on PtnCu(111) surface alloys, which promotes the hydrogen production reaction. The investigation of the local s- and p-projected density states indicates that the extension distribution of state density above the Fermi level plays a important role for the activity of the H on the PtnCu(111) surface alloys.
中文翻译:

Pt原子掺杂Cu(111)表面合金对水离解反应的催化活性
已经提出过渡金属合金作为获得有效和选择性的非均相催化剂用于工业上重要的氢气生产反应的有效策略。已经进行了第一性原理计算,以探索Pt n Cu(111)(n = 1-3)表面合金的催化活性,其中Pt单体,Pt二聚体或Pt三聚体掺入Cu(111)表面层),用于水煤气变换反应(WGSR)。我们的结果表明,掺杂Pt单体的Cu(111)表面对H在Pt n上的迁移显示出低得多的势垒Cu(111)表面合金,可促进制氢反应。对局部s和p投影密度状态的研究表明,费米能级以上状态密度的扩展分布对于H在Pt n Cu(111)表面合金上的活性起着重要作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号