当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enantioconvergent Amination of Racemic Tertiary C–H Bonds
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-11-30 , DOI: 10.1021/jacs.0c11103 Kai Lang 1 , Chaoqun Li 2 , Isaac Kim 1 , X Peter Zhang 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-11-30 , DOI: 10.1021/jacs.0c11103 Kai Lang 1 , Chaoqun Li 2 , Isaac Kim 1 , X Peter Zhang 1
Affiliation
|
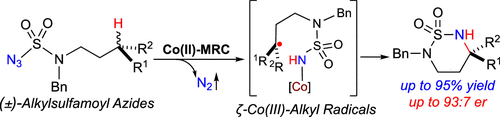
Racemization is considered to be an intrinsic stereochemical feature of free radical chemistry as can be seen in traditional radical halogenation reactions of optically active tertiary C-H bonds. If the facile process of radical racemization could be effectively combined with an ensuing step of bond formation in an enantioselective fashion, then it would give rise to deracemizative functionalization of racemic tertiary C-H bonds for stereoselective construction of chiral molecules bearing quaternary stereocenters. As a demonstration of this unique potential in radical chemistry, we herein report that metalloradical catalysis can be successfully applied to devise Co(II)-based catalytic system for enantioconvergent radical amination of racemic tertiary C(sp3)-H bonds. The key to the success of the radical process is the development of Co(II)-based metalloradical catalyst with fitting steric, electronic, and chiral environments of the D2-symmetric chiral amidoporphyrin as the supporting ligand. The existence of optimal reaction temperature is recognized as an important factor in the realization of the enantioconvergent radical process. Supported by an optimized chiral ligand, the Co(II)-based metalloradical system can effectively catalyze the enantioconvergent 1,6-amination of racemic tertiary C(sp3)-H bonds at the optimal temperature, affording chiral α-tertiary amines in excellent yields with high enantiocontrol of the newly created quaternary stereocenters. Systematic studies, including experiments utilizing optically active deuterium-labeled C-H substrates as a model system, shed light on the underlying mechanistic details of this new catalytic process for enantioconvergent radical C-H amination. The remarkable power to create quaternary stereocenters bearing multiple functionalities from ubiquitous C-H bonds, as showcased with stereoselective construction of bicyclic N-heterocycles, opens the door for future synthetic applications of this new radical technology.
中文翻译:

外消旋叔 C-H 键的对映聚合胺化
外消旋化被认为是自由基化学的固有立体化学特征,这可以在光学活性叔 CH 键的传统自由基卤化反应中看到。如果自由基外消旋的简便过程能够以对映选择性方式与随后的键形成步骤有效结合,那么它将引起外消旋叔 CH 键的去外消旋功能化,用于立体选择性构建带有四元立体中心的手性分子。作为在自由基化学中这种独特潜力的证明,我们在本文中报告说,金属自由基催化可以成功地应用于设计基于 Co(II) 的催化体系,用于外消旋叔 C(sp3)-H 键的对映聚合自由基胺化。自由基过程成功的关键是开发适合 D2 对称手性酰氨基卟啉的空间、电子和手性环境的 Co(II) 基金属基催化剂作为支持配体。最佳反应温度的存在被认为是实现对映聚合自由基过程的重要因素。在优化的手性配体的支持下,基于 Co(II) 的金属自由基体系可以在最佳温度下有效催化外消旋叔 C(sp3)-H 键的对映聚合 1,6-胺化,以优异的产率提供手性 α-叔胺对新创建的四元立体中心具有高对映控制。系统研究,包括使用光学活性氘标记的 CH 底物作为模型系统的实验,阐明了这种对映聚合自由基 CH 胺化的新催化过程的潜在机制细节。从无处不在的 CH 键创建具有多种功能的四元立体中心的非凡能力,正如双环 N-杂环的立体选择性结构所展示的那样,为这种新的自由基技术的未来合成应用打开了大门。
更新日期:2020-11-30
中文翻译:

外消旋叔 C-H 键的对映聚合胺化
外消旋化被认为是自由基化学的固有立体化学特征,这可以在光学活性叔 CH 键的传统自由基卤化反应中看到。如果自由基外消旋的简便过程能够以对映选择性方式与随后的键形成步骤有效结合,那么它将引起外消旋叔 CH 键的去外消旋功能化,用于立体选择性构建带有四元立体中心的手性分子。作为在自由基化学中这种独特潜力的证明,我们在本文中报告说,金属自由基催化可以成功地应用于设计基于 Co(II) 的催化体系,用于外消旋叔 C(sp3)-H 键的对映聚合自由基胺化。自由基过程成功的关键是开发适合 D2 对称手性酰氨基卟啉的空间、电子和手性环境的 Co(II) 基金属基催化剂作为支持配体。最佳反应温度的存在被认为是实现对映聚合自由基过程的重要因素。在优化的手性配体的支持下,基于 Co(II) 的金属自由基体系可以在最佳温度下有效催化外消旋叔 C(sp3)-H 键的对映聚合 1,6-胺化,以优异的产率提供手性 α-叔胺对新创建的四元立体中心具有高对映控制。系统研究,包括使用光学活性氘标记的 CH 底物作为模型系统的实验,阐明了这种对映聚合自由基 CH 胺化的新催化过程的潜在机制细节。从无处不在的 CH 键创建具有多种功能的四元立体中心的非凡能力,正如双环 N-杂环的立体选择性结构所展示的那样,为这种新的自由基技术的未来合成应用打开了大门。

























 京公网安备 11010802027423号
京公网安备 11010802027423号