当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Rationalizing the Decavanadate(V) and Oxidovanadium(IV) Binding to G-Actin and the Competition with Decaniobate(V) and ATP
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2020-11-30 , DOI: 10.1021/acs.inorgchem.0c02971 Giuseppe Sciortino 1, 2 , Manuel Aureliano 3 , Eugenio Garribba 1
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2020-11-30 , DOI: 10.1021/acs.inorgchem.0c02971 Giuseppe Sciortino 1, 2 , Manuel Aureliano 3 , Eugenio Garribba 1
Affiliation
|
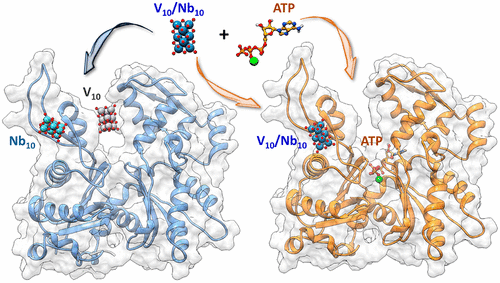
The experimental data collected over the past 15 years on the interaction of decavanadate(V) (V10O286–; V10), a polyoxometalate (POM) with promising anticancer and antibacterial action, with G-actin, were rationalized by using several computational approaches (docking, density functional theory (DFT), and molecular dynamics (MD)). Moreover, a comparison with the isostructural and more stable decaniobate(V) (Nb10O286–; Nb10) was carried out. Four binding sites were identified, named α, β, γ, and δ, the site α being the catalytic nucleotide site located in the cleft of the enzyme at the interface of the subdomains II and IV. It was observed that the site α is preferred by V10, whereas Nb10 is more stable at the site β; this indicates that, differently from other proteins, G-actin could contemporaneously bind the two POMs, whose action would be synergistic. Both decavanadate and decaniobate induce conformational rearrangements in G-actin, larger for V10 than Nb10. Moreover, the binding mode of oxidovanadium(IV) ion, VIVO2+, formed upon the reduction of decavanadate(V) by the –SH groups of accessible cysteine residues, is also found in the catalytic site α with (His161, Asp154) coordination; this adduct overlaps significantly with the region where ATP is bound, accounting for the competition between V10 and its reduction product VIVO2+ with ATP, as previously observed by EPR spectroscopy. Finally, the competition with ATP was rationalized: since decavanadate prefers the nucleotide site α, Ca2+-ATP displaces V10 from this site, while the competition is less important for Nb10 because this POM shows a higher affinity for β than for site α. A relevant consequence of this paper is that other metallodrug–protein systems, in the absence or presence of eventual inhibitors and/or competition with molecules of the organism, could be studied with the same approach, suggesting important elements for an explanation of the biological data and a rational drug design.
中文翻译:

合理化十钒酸盐(V)和氧化钒(IV)与G-肌动蛋白的结合以及与十价铌酸盐(V)和ATP的竞争
过去15年中收集的关于十钒酸盐(V)(V 10 O 28 6– ; V 10),具有良好抗癌和抗菌作用的多金属氧酸盐(POM)与G-肌动蛋白的相互作用的实验数据通过几种计算方法(对接,密度泛函理论(DFT)和分子动力学(MD))。此外,与同构且更稳定的癸二酸盐(V)(Nb 10 O 28 6– ; Nb 10)进行。鉴定出四个结合位点,命名为α,β,γ和δ,位点α是位于亚结构域II和IV的界面处的酶的裂口中的催化核苷酸位点。观察到,V 10优选α位,而β10 Nb 10更稳定。这表明,与其他蛋白质不同,G-肌动蛋白可以同时结合两个POM,它们的作用是协同的。十vanadate和decaniobate均可在G-肌动蛋白中诱导构象重排,对于V 10而言,它比Nb 10大。此外,oxidovanadium(IV)离子的结合模式,V IV ö 2+,在可触及的半胱氨酸残基的–SH基团将十烷钒酸盐(V)还原后形成的,也发现在具有(His161,Asp154)配位的催化位点α中;该加合物与结合ATP的区域显着重叠,这说明了V 10及其还原产物V IV O 2+与ATP之间的竞争,如先前通过EPR光谱观察到的。最终,与ATP的竞争得以合理化:由于十钒酸盐偏爱核苷酸位点α,因此Ca 2+ -ATP取代了该位点上的V 10,而竞争对Nb 10的重要性则降低了因为该POM对β的亲和力高于对位α的亲和力。本文的一个相关结果是,在没有或存在最终抑制剂和/或与生物分子竞争的情况下,其他金属药物-蛋白质系统也可以用相同的方法进行研究,为解释生物学数据提供了重要的提示和合理的药物设计。
更新日期:2021-01-04
中文翻译:

合理化十钒酸盐(V)和氧化钒(IV)与G-肌动蛋白的结合以及与十价铌酸盐(V)和ATP的竞争
过去15年中收集的关于十钒酸盐(V)(V 10 O 28 6– ; V 10),具有良好抗癌和抗菌作用的多金属氧酸盐(POM)与G-肌动蛋白的相互作用的实验数据通过几种计算方法(对接,密度泛函理论(DFT)和分子动力学(MD))。此外,与同构且更稳定的癸二酸盐(V)(Nb 10 O 28 6– ; Nb 10)进行。鉴定出四个结合位点,命名为α,β,γ和δ,位点α是位于亚结构域II和IV的界面处的酶的裂口中的催化核苷酸位点。观察到,V 10优选α位,而β10 Nb 10更稳定。这表明,与其他蛋白质不同,G-肌动蛋白可以同时结合两个POM,它们的作用是协同的。十vanadate和decaniobate均可在G-肌动蛋白中诱导构象重排,对于V 10而言,它比Nb 10大。此外,oxidovanadium(IV)离子的结合模式,V IV ö 2+,在可触及的半胱氨酸残基的–SH基团将十烷钒酸盐(V)还原后形成的,也发现在具有(His161,Asp154)配位的催化位点α中;该加合物与结合ATP的区域显着重叠,这说明了V 10及其还原产物V IV O 2+与ATP之间的竞争,如先前通过EPR光谱观察到的。最终,与ATP的竞争得以合理化:由于十钒酸盐偏爱核苷酸位点α,因此Ca 2+ -ATP取代了该位点上的V 10,而竞争对Nb 10的重要性则降低了因为该POM对β的亲和力高于对位α的亲和力。本文的一个相关结果是,在没有或存在最终抑制剂和/或与生物分子竞争的情况下,其他金属药物-蛋白质系统也可以用相同的方法进行研究,为解释生物学数据提供了重要的提示和合理的药物设计。



























 京公网安备 11010802027423号
京公网安备 11010802027423号