当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Sterically Demanding 8-(Diphenylphosphino)quinoline Complexes of Group 10 Metal(II): Synthesis, Crystal Structures, and Properties in Solution
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2020-11-29 , DOI: 10.1021/acs.inorgchem.0c02706 Masatoshi Mori 1 , Yukinari Sunatsuki 1 , Takayoshi Suzuki 1, 2
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2020-11-29 , DOI: 10.1021/acs.inorgchem.0c02706 Masatoshi Mori 1 , Yukinari Sunatsuki 1 , Takayoshi Suzuki 1, 2
Affiliation
|
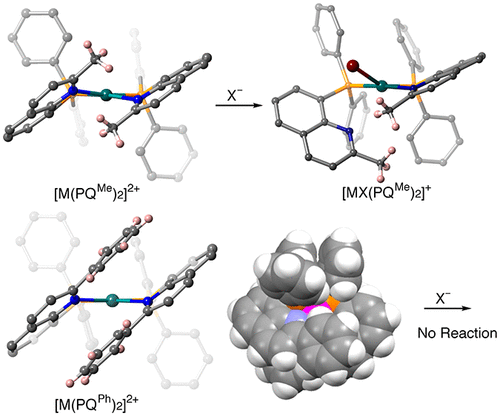
Several series of platinum(II), palladium(II), and nickel(II) complexes bearing 8-(diphenylphosphino)quinoline (PQH) or its 2-methyl or 2-phenyl derivatives (PQMe or PQPh) were synthesized, and their crystal structures and behaviors in solution were investigated. Most of the complexes [M(PQR)2]X2 (MII = PtII, PdII, or NiII; R = H, Me or Ph; X = monoanionic ions) characterized in this study have an approximately square-planar coordination geometry with two bidentate P,N-chelating or monodentate P-donating quinolylphosphine ligands in the cis(P,P) configuration. A large steric requirement from the Me or Ph substituent introduced at the 2-position of the quinoline ring gives the resulting complexes severe distortion. The PtII and PdII complex cations maintained the square-planar coordination geometry, but the MII center was displaced from the chelating ligand plane. This bending of the chelate coordination makes the M–N(quinoline) bond weaker, as demonstrated by the longer M–N bonds. In accord with the bond weakening, the partial dissociation of the PQH or PQMe chelates by substitution with halide anions were observed using UV–vis spectroscopy and X-ray crystallography. In contrast, the PQPh complexes were stable in solution toward the addition of halide anions; the intramolecular π–π stacking interaction between the coordinating quinolyl and the 2-substituted phenyl rings protects the MII center from nucleophilic attack. In the corresponding NiII complexes, the steric congestion arising from the mutually cis-positioned PQR ligands resulted in a large tetrahedral distortion around the NiII center. However, the intramolecular π–π stacking interaction was still effective in the PQPh complex, and this interaction can explain some unusual robustness and electrochemical properties of the NiII–PQPh complex.
中文翻译:

立体要求的第10组金属(II)的8-(二苯基膦基)喹啉配合物:溶液的合成,晶体结构和性质
合成了几个带有8-(二苯基膦基)喹啉(PQ H)或其2-甲基或2-苯基衍生物(PQ Me或PQ Ph)的铂(II),钯(II)和镍(II)配合物系列,研究了它们的晶体结构和在溶液中的行为。在这项研究中表征的大多数络合物[M(PQ R)2 ] X 2(M II = Pt II,Pd II或Ni II; R = H,Me或Ph; X =单阴离子)具有两个双齿P,N螯合或单齿P的平面配位几何-顺式(P,P)构型的供体喹啉基膦配体。喹啉环2位上引入的Me或Ph取代基对空间的要求很高,导致所得络合物严重变形。Pt II和Pd II配位阳离子保持方形平面配位几何,但M II中心从螯合配体平面移开。螯合配位基团的这种弯曲使M–N(喹啉)键变弱,如更长的M–N键所示。随着键的减弱,PQ H或PQ Me的部分解离通过紫外可见光谱和X射线晶体学观察到被卤化物阴离子取代的螯合剂。相反,PQ Ph络合物在溶液中对添加卤化物阴离子稳定;配位的喹啉基和2-取代的苯环之间的分子内π-π堆积相互作用可保护M II中心免受亲核攻击。在相应的Ni II配合物中,由相互顺式定位的PQ R配体引起的空间拥挤导致Ni II中心周围出现大的四面体形变。但是,分子内π–π堆积相互作用在PQ Ph中仍然有效这种相互作用可以解释Ni II -PQ Ph配合物的一些异常坚固性和电化学性质。
更新日期:2020-12-21
中文翻译:

立体要求的第10组金属(II)的8-(二苯基膦基)喹啉配合物:溶液的合成,晶体结构和性质
合成了几个带有8-(二苯基膦基)喹啉(PQ H)或其2-甲基或2-苯基衍生物(PQ Me或PQ Ph)的铂(II),钯(II)和镍(II)配合物系列,研究了它们的晶体结构和在溶液中的行为。在这项研究中表征的大多数络合物[M(PQ R)2 ] X 2(M II = Pt II,Pd II或Ni II; R = H,Me或Ph; X =单阴离子)具有两个双齿P,N螯合或单齿P的平面配位几何-顺式(P,P)构型的供体喹啉基膦配体。喹啉环2位上引入的Me或Ph取代基对空间的要求很高,导致所得络合物严重变形。Pt II和Pd II配位阳离子保持方形平面配位几何,但M II中心从螯合配体平面移开。螯合配位基团的这种弯曲使M–N(喹啉)键变弱,如更长的M–N键所示。随着键的减弱,PQ H或PQ Me的部分解离通过紫外可见光谱和X射线晶体学观察到被卤化物阴离子取代的螯合剂。相反,PQ Ph络合物在溶液中对添加卤化物阴离子稳定;配位的喹啉基和2-取代的苯环之间的分子内π-π堆积相互作用可保护M II中心免受亲核攻击。在相应的Ni II配合物中,由相互顺式定位的PQ R配体引起的空间拥挤导致Ni II中心周围出现大的四面体形变。但是,分子内π–π堆积相互作用在PQ Ph中仍然有效这种相互作用可以解释Ni II -PQ Ph配合物的一些异常坚固性和电化学性质。


























 京公网安备 11010802027423号
京公网安备 11010802027423号