当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
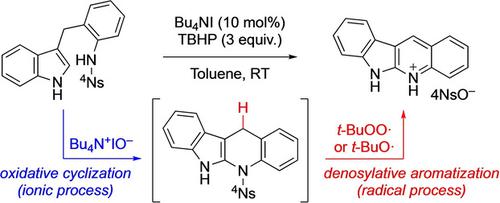
I+/TBHP Catalysis For Tandem Oxidative Cyclization To Indolo[2,3‐b]quinolines
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2020-11-30 , DOI: 10.1002/ajoc.202000570 Muhammet Uyanik 1 , Hiroki Tanaka 1 , Kazuaki Ishihara 1
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2020-11-30 , DOI: 10.1002/ajoc.202000570 Muhammet Uyanik 1 , Hiroki Tanaka 1 , Kazuaki Ishihara 1
Affiliation
|
We report a chemoselective tandem oxidative cyclization/aromatization of indole derivatives tethered to aniline sulfonamides using catalytic amount of tetrabutylammonium iodide in the presence of tert‐butyl hydroperoxide (TBHP) as an oxidant under nearly neutral conditions at room temperature. The corresponding indolo[2,3‐b]quinolines were obtained as sulfonate salts, which could be easily isolated in analytically pure form via only a simple filtration of the crude reaction mixture. The natural product quinindoline could be easily obtained after basic work‐up of the sulfonate salt. Control experiments revealed that both ionic and radical active species could be generated in situ under mild conditions for the corresponding oxidative transformations to proceed in a chemoselective manner.
中文翻译:

I + / TBHP催化串联氧化环化吲哚[2,3-b]喹啉
我们报告了在室温接近中性的条件下,在叔丁基氢过氧化物(TBHP)作为氧化剂存在的情况下,使用催化量的碘化四丁基碘化铵,将连接到苯胺磺酰胺的吲哚衍生物进行化学选择性串联氧化环化/芳香化。相应的吲哚并[2,3- b ]喹啉以磺酸盐形式获得,只需简单过滤粗反应混合物即可轻松分离为分析纯形式。磺酸盐经过基本处理后,即可轻松获得天然产物喹啉。对照实验表明,离子和自由基活性物质均可原位产生 在温和条件下,以化学选择性方式进行相应的氧化转化。
更新日期:2021-01-18
中文翻译:

I + / TBHP催化串联氧化环化吲哚[2,3-b]喹啉
我们报告了在室温接近中性的条件下,在叔丁基氢过氧化物(TBHP)作为氧化剂存在的情况下,使用催化量的碘化四丁基碘化铵,将连接到苯胺磺酰胺的吲哚衍生物进行化学选择性串联氧化环化/芳香化。相应的吲哚并[2,3- b ]喹啉以磺酸盐形式获得,只需简单过滤粗反应混合物即可轻松分离为分析纯形式。磺酸盐经过基本处理后,即可轻松获得天然产物喹啉。对照实验表明,离子和自由基活性物质均可原位产生 在温和条件下,以化学选择性方式进行相应的氧化转化。


























 京公网安备 11010802027423号
京公网安备 11010802027423号