当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A Concise Total Synthesis of (−)‐Berkelic Acid
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2020-11-30 , DOI: 10.1002/anie.202014660 Hong‐Gang Cheng 1 , Zhenjie Yang 1 , Ruiming Chen 1 , Liming Cao 1 , Wen‐Yan Tong 2 , Qiang Wei 1 , Qingqing Wang 1 , Chenggui Wu 1 , Shuanglin Qu 2 , Qianghui Zhou 1, 3
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2020-11-30 , DOI: 10.1002/anie.202014660 Hong‐Gang Cheng 1 , Zhenjie Yang 1 , Ruiming Chen 1 , Liming Cao 1 , Wen‐Yan Tong 2 , Qiang Wei 1 , Qingqing Wang 1 , Chenggui Wu 1 , Shuanglin Qu 2 , Qianghui Zhou 1, 3
Affiliation
|
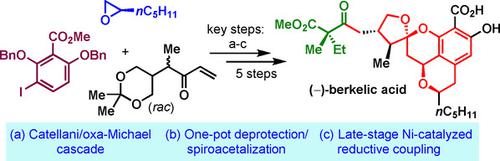
Reported here is a concise total synthesis of (−)‐berkelic acid in eight linear steps. This synthesis features a Catellani reaction/oxa‐Michael cascade for the construction of the isochroman scaffold, a one‐pot deprotection/spiroacetalization operation for the formation of the tetracyclic core structure, and a late‐stage Ni‐catalyzed reductive coupling for the introduction of the lateral chain. Notably, four stereocenters are established from a single existing chiral center with excellent stereocontrol during the deprotection/spiroacetalization process. Stereocontrol of the intriguing deprotection/spiroacetalization process is supported by DFT calculations.
中文翻译:

简明全合成(-)-铍酸
此处报告的是八个线性步骤的简明全合成(-)-伯酸。该合成的特征是用于构建异色满骨架的Catellani反应/ oxa-Michael级联反应,用于形成四环核心结构的一锅脱保护/螺缩醛化操作以及用于引入Ni的后期Ni催化还原偶联。横向链。值得注意的是,在脱保护/螺缩醛化过程中,从一个现有的手性中心建立了四个立体中心,并具有出色的立体控制。DFT计算支持对有趣的脱保护/螺缩醛化过程的立体控制。
更新日期:2020-11-30
中文翻译:

简明全合成(-)-铍酸
此处报告的是八个线性步骤的简明全合成(-)-伯酸。该合成的特征是用于构建异色满骨架的Catellani反应/ oxa-Michael级联反应,用于形成四环核心结构的一锅脱保护/螺缩醛化操作以及用于引入Ni的后期Ni催化还原偶联。横向链。值得注意的是,在脱保护/螺缩醛化过程中,从一个现有的手性中心建立了四个立体中心,并具有出色的立体控制。DFT计算支持对有趣的脱保护/螺缩醛化过程的立体控制。


























 京公网安备 11010802027423号
京公网安备 11010802027423号