当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Chemical Control of Spin‐Orbit Coupling and Charge Transfer in Vacancy‐Ordered Ruthenium(IV) Halide Perovskites
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2020-11-27 , DOI: 10.1002/anie.202013383 Pratap Vishnoi 1 , Julia L. Zuo 1 , Joya A. Cooley 2 , Linus Kautzsch 1 , Alejandra Gómez‐Torres 3 , Jesse Murillo 3 , Skye Fortier 3 , Stephen D. Wilson 1 , Ram Seshadri 1 , Anthony K. Cheetham 1, 4
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2020-11-27 , DOI: 10.1002/anie.202013383 Pratap Vishnoi 1 , Julia L. Zuo 1 , Joya A. Cooley 2 , Linus Kautzsch 1 , Alejandra Gómez‐Torres 3 , Jesse Murillo 3 , Skye Fortier 3 , Stephen D. Wilson 1 , Ram Seshadri 1 , Anthony K. Cheetham 1, 4
Affiliation
|
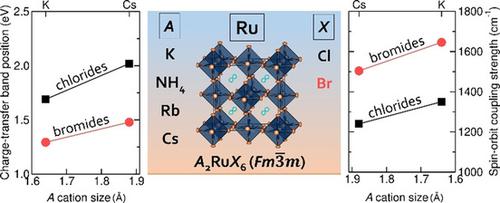
Vacancy‐ordered double perovskites are attracting significant attention due to their chemical diversity and interesting optoelectronic properties. With a view to understanding both the optical and magnetic properties of these compounds, two series of RuIV halides are presented; A2RuCl6 and A2RuBr6, where A is K, NH4, Rb or Cs. We show that the optical properties and spin‐orbit coupling (SOC) behavior can be tuned through changing the A cation and the halide. Within a series, the energy of the ligand‐to‐metal charge transfer increases as the unit cell expands with the larger A cation, and the band gaps are higher for the respective chlorides than for the bromides. The magnetic moments of the systems are temperature dependent due to a non‐magnetic ground state with Jeff=0 caused by SOC. Ru‐X covalency, and consequently, the delocalization of metal d‐electrons, result in systematic trends of the SOC constants due to variations in the A cation and the halide anion.
中文翻译:

空位有序的卤化钙钛矿中自旋轨道耦合和电荷转移的化学控制
空位排序的钙钛矿由于其化学多样性和有趣的光电特性而备受关注。为了理解这些化合物的光学和磁性,提出了两个系列的Ru IV卤化物。A 2 RuCl 6和A 2 RuBr 6,其中A为K,NH 4,Rb或Cs。我们表明,可以通过改变A阳离子和卤化物来调节光学性质和自旋轨道耦合(SOC)行为。在一个系列中,配体到金属的电荷转移能量随着晶胞的扩大而增加,而更大的A离子,并且相应的氯化物的带隙高于溴化物。由于SOC导致J eff = 0的非磁性基态,系统的磁矩与温度有关。由于A阳离子和卤化物阴离子的变化,Ru‐ X的共价关系以及金属d电子的离域化导致SOC常数的系统趋势。
更新日期:2020-11-27
中文翻译:

空位有序的卤化钙钛矿中自旋轨道耦合和电荷转移的化学控制
空位排序的钙钛矿由于其化学多样性和有趣的光电特性而备受关注。为了理解这些化合物的光学和磁性,提出了两个系列的Ru IV卤化物。A 2 RuCl 6和A 2 RuBr 6,其中A为K,NH 4,Rb或Cs。我们表明,可以通过改变A阳离子和卤化物来调节光学性质和自旋轨道耦合(SOC)行为。在一个系列中,配体到金属的电荷转移能量随着晶胞的扩大而增加,而更大的A离子,并且相应的氯化物的带隙高于溴化物。由于SOC导致J eff = 0的非磁性基态,系统的磁矩与温度有关。由于A阳离子和卤化物阴离子的变化,Ru‐ X的共价关系以及金属d电子的离域化导致SOC常数的系统趋势。


























 京公网安备 11010802027423号
京公网安备 11010802027423号