当前位置:
X-MOL 学术
›
Surf. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The interaction of several fluorinated ionic liquids on the LiF(001) surface
Surfaces and Interfaces ( IF 6.2 ) Pub Date : 2021-02-01 , DOI: 10.1016/j.surfin.2020.100836 Annelisa S. Rigoni , Michael Breedon , Gavin E. Collis , Michelle J.S. Spencer
Surfaces and Interfaces ( IF 6.2 ) Pub Date : 2021-02-01 , DOI: 10.1016/j.surfin.2020.100836 Annelisa S. Rigoni , Michael Breedon , Gavin E. Collis , Michelle J.S. Spencer
|
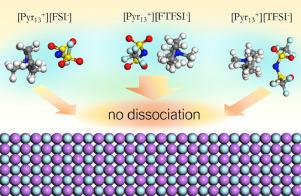
ABSTRACT LiF is a well-known constituent of the solid electrolyte interphase (SEI) layer in lithium metal batteries, which spontaneously forms in the presence of electrolytes and additives. The subsequent interaction of the electrolyte with this component at the atomic scale, however, is largely unreported. In this work, density functional theory (DFT) calculations were used to examine the interaction of several ILs, specifically 1-methyl-1-propylpyrrolidinium cation (Pyr13+) paired with the fluorinated anions bis(fluorosulfonyl)imide (FSI−), fluorosulfonyl-(trifluoromethanesulfonyl)imide (FTFSI−), and bis(trifluoromethylsulfonyl)imide (TFSI−) on a LiF(001) surface, to detail their adsorption characteristics. Each IL was shown to adsorb onto the LiF surface in different orientations via the anion O and F atoms and the cation H atoms, with binding energies ranging from -1.54 to -0.22 eV. These surface binding energies are significantly weaker than the corresponding anion-cation interaction energy. The thermal stability of each IL on the LiF surface was explored via AIMD simulations at 428 K, revealing that each of the examined IL pairs did not dissociate on the surface within the examined simulation time. This work further reinforces the stability of fluorinated ionic liquids on a LiF interface and provides a highly detailed account of the adsorption process.
中文翻译:

几种氟化离子液体在 LiF(001) 表面的相互作用
摘要 LiF 是锂金属电池中固体电解质中间相 (SEI) 层的一种众所周知的成分,它在电解质和添加剂的存在下自发形成。然而,电解质与该组分在原子尺度上的后续相互作用在很大程度上未被报道。在这项工作中,密度泛函理论 (DFT) 计算用于检查几种 IL 的相互作用,特别是 1-甲基-1-丙基吡咯烷阳离子 (Pyr13+) 与氟化阴离子双(氟磺酰基)酰亚胺(FSI-)、氟磺酰基- LiF(001)表面上的(三氟甲磺酰基)酰亚胺(FTFSI-)和双(三氟甲基磺酰基)酰亚胺(TFSI-),以详细说明它们的吸附特性。每个 IL 都通过阴离子 O 和 F 原子以及阳离子 H 原子以不同的方向吸附到 LiF 表面,结合能范围从 -1.54 到 -0.22 eV。这些表面结合能明显弱于相应的阴离子-阳离子相互作用能。通过 AIMD 模拟在 428 K 下探索了 LiF 表面上每个 IL 的热稳定性,表明每个检查的 IL 对在检查的模拟时间内没有在表面解离。这项工作进一步增强了氟化离子液体在 LiF 界面上的稳定性,并提供了对吸附过程的高度详细说明。揭示每个检查的 IL 对在检查的模拟时间内没有在表面解离。这项工作进一步增强了氟化离子液体在 LiF 界面上的稳定性,并提供了对吸附过程的高度详细说明。揭示每个检查的 IL 对在检查的模拟时间内没有在表面解离。这项工作进一步增强了氟化离子液体在 LiF 界面上的稳定性,并提供了对吸附过程的高度详细说明。
更新日期:2021-02-01
中文翻译:

几种氟化离子液体在 LiF(001) 表面的相互作用
摘要 LiF 是锂金属电池中固体电解质中间相 (SEI) 层的一种众所周知的成分,它在电解质和添加剂的存在下自发形成。然而,电解质与该组分在原子尺度上的后续相互作用在很大程度上未被报道。在这项工作中,密度泛函理论 (DFT) 计算用于检查几种 IL 的相互作用,特别是 1-甲基-1-丙基吡咯烷阳离子 (Pyr13+) 与氟化阴离子双(氟磺酰基)酰亚胺(FSI-)、氟磺酰基- LiF(001)表面上的(三氟甲磺酰基)酰亚胺(FTFSI-)和双(三氟甲基磺酰基)酰亚胺(TFSI-),以详细说明它们的吸附特性。每个 IL 都通过阴离子 O 和 F 原子以及阳离子 H 原子以不同的方向吸附到 LiF 表面,结合能范围从 -1.54 到 -0.22 eV。这些表面结合能明显弱于相应的阴离子-阳离子相互作用能。通过 AIMD 模拟在 428 K 下探索了 LiF 表面上每个 IL 的热稳定性,表明每个检查的 IL 对在检查的模拟时间内没有在表面解离。这项工作进一步增强了氟化离子液体在 LiF 界面上的稳定性,并提供了对吸附过程的高度详细说明。揭示每个检查的 IL 对在检查的模拟时间内没有在表面解离。这项工作进一步增强了氟化离子液体在 LiF 界面上的稳定性,并提供了对吸附过程的高度详细说明。揭示每个检查的 IL 对在检查的模拟时间内没有在表面解离。这项工作进一步增强了氟化离子液体在 LiF 界面上的稳定性,并提供了对吸附过程的高度详细说明。


























 京公网安备 11010802027423号
京公网安备 11010802027423号