Chemistry and Physics of Lipids ( IF 3.4 ) Pub Date : 2020-11-27 , DOI: 10.1016/j.chemphyslip.2020.105022 William T Heller 1
|
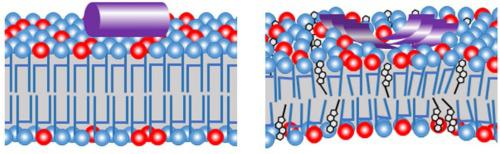
Viruses have evolved a variety of ways for delivering their genetic cargo to a target cell. One mechanism relies on a short sequence from a protein of the virus that is referred to as a fusion peptide. In some cases, the isolated fusion peptide is also capable of causing membranes to fuse. Infection by HIV-1 involves the 23 amino acid N-terminal sequence of its gp41 envelope protein, which is capable of causing membranes to fuse by itself, but the mechanism by which it does so is not fully understood. Here, a variant of the gp41 fusion peptide that does not strongly promote fusion was studied in the presence of vesicles composed of a mixture of unsaturated lipids and cholesterol by small-angle neutron scattering and circular dichroism spectroscopy to improve the understanding of the mechanism that drives vesicle fusion. The peptide concentration and cholesterol content govern both the peptide conformation and its impact on the bilayer structure. The results indicate that the mechanism that drives vesicle fusion by the peptide is a strong distortion of the bilayer structure by the peptide when it adopts the β-sheet conformation.
中文翻译:

驱动病毒融合肽作用的物理机制的小角度中子散射研究
病毒已经进化出多种方式将其遗传物质传递到目标细胞。一种机制依赖于来自病毒蛋白质的短序列,称为融合肽。在某些情况下,分离的融合肽也能够导致膜融合。HIV-1 感染涉及其 gp41 包膜蛋白的 23 个氨基酸 N 末端序列,该蛋白能够导致膜自身融合,但其机制尚不完全清楚。在这里,在由不饱和脂质和胆固醇的混合物组成的囊泡存在的情况下,通过小角中子散射和圆二色光谱研究了不强烈促进融合的 gp41 融合肽的变体,以提高对驱动机制的理解囊泡融合。肽浓度和胆固醇含量控制着肽构象及其对双层结构的影响。结果表明,当肽采用β-折叠构象时,肽驱动囊泡融合的机制是肽对双层结构的强烈扭曲。

























 京公网安备 11010802027423号
京公网安备 11010802027423号