当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
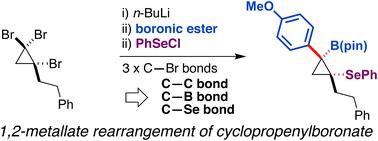
Synthesis of functionalized cyclopropylboronic esters based on a 1,2-metallate rearrangement of cyclopropenylboronate
Chemical Communications ( IF 4.9 ) Pub Date : 2020-11-18 , DOI: 10.1039/d0cc07134j Haruki Mizoguchi 1, 2, 3, 4 , Masaya Seriu 1, 2, 3, 4 , Akira Sakakura 1, 2, 3, 4
Chemical Communications ( IF 4.9 ) Pub Date : 2020-11-18 , DOI: 10.1039/d0cc07134j Haruki Mizoguchi 1, 2, 3, 4 , Masaya Seriu 1, 2, 3, 4 , Akira Sakakura 1, 2, 3, 4
Affiliation
|
A procedure converting tribromocyclopropane to densely functionalized β-selenocyclopropylboronic ester using the 1,2-metallate rearrangement of a boron ate-complex has been developed. Treatment of an in situ-generated cyclopropenylboronic ester ate-complex with phenylselenenyl chloride triggered stereospecific rearrangement to produce functionalized cyclopropanes. DFT calculations for 1,2-metallate rearrangement suggested that the reaction proceeds through a seleniranium intermediate.
中文翻译:

基于环丙烯基硼酸酯的1,2-金属盐重排合成功能化环丙基硼酸酯
已经开发出使用硼酸盐配合物的1,2-金属盐重排将三溴环丙烷转化为高官能度的β-硒环丙基硼酸酯的方法。用苯基硒烯基氯处理原位产生的环丙烯基硼酸酯产物复合物引发立体有规重排,以产生官能化的环丙烷。DFT对1,2-金属盐重排的计算表明,反应是通过硒中间体进行的。
更新日期:2020-11-27
中文翻译:

基于环丙烯基硼酸酯的1,2-金属盐重排合成功能化环丙基硼酸酯
已经开发出使用硼酸盐配合物的1,2-金属盐重排将三溴环丙烷转化为高官能度的β-硒环丙基硼酸酯的方法。用苯基硒烯基氯处理原位产生的环丙烯基硼酸酯产物复合物引发立体有规重排,以产生官能化的环丙烷。DFT对1,2-金属盐重排的计算表明,反应是通过硒中间体进行的。


























 京公网安备 11010802027423号
京公网安备 11010802027423号