Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Deuterium effects on the vibrational circular dichroism spectra of flavanone
Chirality ( IF 2 ) Pub Date : 2020-11-26 , DOI: 10.1002/chir.23289 Marcelo A Muñoz 1 , Pedro Joseph-Nathan 2
Chirality ( IF 2 ) Pub Date : 2020-11-26 , DOI: 10.1002/chir.23289 Marcelo A Muñoz 1 , Pedro Joseph-Nathan 2
Affiliation
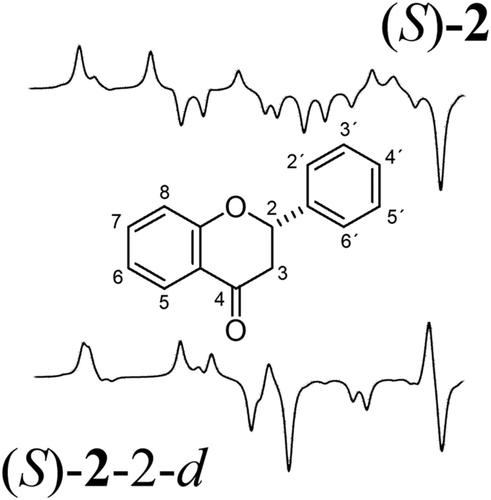
|
The minimum chemical modification that can be incorporated into an organic molecule is the replacement of a hydrogen atom for a deuterium atom. This change is not altering the pharmacological properties of a molecule, although it provides the possibility of making specific spectroscopic evaluations. Thus, in the present study, we explore how a stereogenic center is influenced by such an isotopic labeling. The studies were conducted on both enantiomers of flavanone (1 and 2) which is the parent molecule of a large group of pharmacologically active natural occurring secondary metabolites. Flavanone comprised 12 carbon atoms forming two benzene rings, a carbonyl group, an ethereal oxygen atom, a methylene group, and only one C–H stereogenic center, so it seems to be an ideal candidate for such studies. Density functional theory (DFT) calculations were used for the accurate prediction of vibrational circular dichroism (VCD) spectra of (R)‐(3) and (S)‐flavanone‐2‐d (4), of (R)‐(5) and (S)‐flavanone‐3,3‐d2 (6), and of (R)‐(7) and (S)‐flavanone‐2′,3′,4′,5′,6′‐d5 (8). To gain compounds that provide experimental VCD spectra for comparative purposes, the calculated spectra of both enantiomers of the corresponding flavanones, obtained after HPLC separation of the racemates by means of a chiral column, were contrasted, thereby revealing excellent agreements when using the CompareVOA software. In addition, the VCD spectra of both unlabeled enantiomeric flavanones (1 and 2) were also compared to the labeled molecules, revealing that the VCD spectra show significant variations induced by the deuterium incorporation.
中文翻译:

氘对黄烷酮振动圆二色光谱的影响
可以掺入有机分子的最小化学修饰是用氢原子替换氘原子。这种变化不会改变分子的药理特性,尽管它提供了进行特定光谱评估的可能性。因此,在本研究中,我们探讨了立体中心如何受到这种同位素标记的影响。对黄烷酮的两种对映异构体(1和2) 它是一大类具有药理活性的天然次级代谢物的母体分子。黄烷酮由 12 个碳原子组成,形成两个苯环、一个羰基、一个醚氧原子、一个亚甲基,并且只有一个 C-H 立体中心,因此它似乎是此类研究的理想候选者。密度泛函理论 (DFT) 计算用于准确预测 ( R )-( 3 ) 和 ( S )-黄烷酮-2- d ( 4 ), ( R )-( 5 )的振动圆二色性 (VCD) 光谱) 和 ( S )-黄烷酮-3,3- d 2 ( 6 ), 和 ( R)-( 7 ) 和 ( S )-黄烷酮-2',3',4',5',6'- d 5 ( 8 )。为了获得提供实验 VCD 光谱的化合物以供比较,通过 HPLC 分离外消旋物后通过手性柱获得的相应黄烷酮的两种对映异构体的计算光谱进行了对比,从而在使用 Compare VOA软件时显示出极好的一致性. 此外,未标记的对映体黄烷酮(1和2)的 VCD 光谱也与标记的分子进行了比较,表明 VCD 光谱显示出由氘掺入引起的显着变化。
更新日期:2021-01-20
中文翻译:

氘对黄烷酮振动圆二色光谱的影响
可以掺入有机分子的最小化学修饰是用氢原子替换氘原子。这种变化不会改变分子的药理特性,尽管它提供了进行特定光谱评估的可能性。因此,在本研究中,我们探讨了立体中心如何受到这种同位素标记的影响。对黄烷酮的两种对映异构体(1和2) 它是一大类具有药理活性的天然次级代谢物的母体分子。黄烷酮由 12 个碳原子组成,形成两个苯环、一个羰基、一个醚氧原子、一个亚甲基,并且只有一个 C-H 立体中心,因此它似乎是此类研究的理想候选者。密度泛函理论 (DFT) 计算用于准确预测 ( R )-( 3 ) 和 ( S )-黄烷酮-2- d ( 4 ), ( R )-( 5 )的振动圆二色性 (VCD) 光谱) 和 ( S )-黄烷酮-3,3- d 2 ( 6 ), 和 ( R)-( 7 ) 和 ( S )-黄烷酮-2',3',4',5',6'- d 5 ( 8 )。为了获得提供实验 VCD 光谱的化合物以供比较,通过 HPLC 分离外消旋物后通过手性柱获得的相应黄烷酮的两种对映异构体的计算光谱进行了对比,从而在使用 Compare VOA软件时显示出极好的一致性. 此外,未标记的对映体黄烷酮(1和2)的 VCD 光谱也与标记的分子进行了比较,表明 VCD 光谱显示出由氘掺入引起的显着变化。


























 京公网安备 11010802027423号
京公网安备 11010802027423号