当前位置:
X-MOL 学术
›
Clin. Transl. Immunol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Human‐engineered Treg‐like cells suppress FOXP3‐deficient T cells but preserve adaptive immune responses in vivo
Clinical & Translational Immunology ( IF 5.8 ) Pub Date : 2020-11-25 , DOI: 10.1002/cti2.1214 Yohei Sato 1 , Laura Passerini 2 , Brian D Piening 3 , Molly Javier Uyeda 1, 4 , Marianne Goodwin 1 , Silvia Gregori 2 , Michael P Snyder 5 , Alice Bertaina 1 , Maria-Grazia Roncarolo 1, 4, 6 , Rosa Bacchetta 1, 6
Clinical & Translational Immunology ( IF 5.8 ) Pub Date : 2020-11-25 , DOI: 10.1002/cti2.1214 Yohei Sato 1 , Laura Passerini 2 , Brian D Piening 3 , Molly Javier Uyeda 1, 4 , Marianne Goodwin 1 , Silvia Gregori 2 , Michael P Snyder 5 , Alice Bertaina 1 , Maria-Grazia Roncarolo 1, 4, 6 , Rosa Bacchetta 1, 6
Affiliation
|
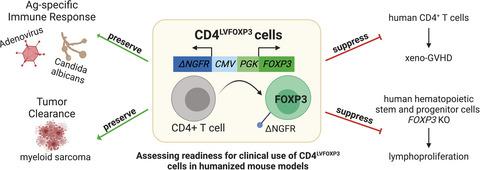
Genetic or acquired defects in FOXP3+ regulatory T cells (Tregs) play a key role in many immune‐mediated diseases including immune dysregulation polyendocrinopathy, enteropathy, X‐linked (IPEX) syndrome. Previously, we demonstrated CD4+ T cells from healthy donors and IPEX patients can be converted into functional Treg‐like cells by lentiviral transfer of FOXP3 (CD4LVFOXP3). These CD4LVFOXP3 cells have potent regulatory function, suggesting their potential as an innovative therapeutic. Here, we present molecular and preclinical in vivo data supporting CD4LVFOXP3 cell clinical progression.
中文翻译:

人类工程改造的 Treg 样细胞抑制 FOXP3 缺陷型 T 细胞,但在体内保留适应性免疫反应
FOXP3 +调节性 T 细胞 (Tregs) 的遗传或获得性缺陷在许多免疫介导疾病中发挥关键作用,包括免疫失调多内分泌病、肠病、X 连锁 (IPEX) 综合征。此前,我们证明了来自健康供体和 IPEX 患者的 CD4 + T 细胞可以通过FOXP3(CD4 LVFOXP3)的慢病毒转移转化为功能性 Treg 样细胞。这些 CD4 LVFOXP3细胞具有强大的调节功能,表明它们具有作为创新疗法的潜力。在这里,我们提供了支持 CD4 LVFOXP3细胞临床进展的分子和临床前体内数据。
更新日期:2020-11-27
中文翻译:

人类工程改造的 Treg 样细胞抑制 FOXP3 缺陷型 T 细胞,但在体内保留适应性免疫反应
FOXP3 +调节性 T 细胞 (Tregs) 的遗传或获得性缺陷在许多免疫介导疾病中发挥关键作用,包括免疫失调多内分泌病、肠病、X 连锁 (IPEX) 综合征。此前,我们证明了来自健康供体和 IPEX 患者的 CD4 + T 细胞可以通过FOXP3(CD4 LVFOXP3)的慢病毒转移转化为功能性 Treg 样细胞。这些 CD4 LVFOXP3细胞具有强大的调节功能,表明它们具有作为创新疗法的潜力。在这里,我们提供了支持 CD4 LVFOXP3细胞临床进展的分子和临床前体内数据。


























 京公网安备 11010802027423号
京公网安备 11010802027423号