当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mixed Noble‐Gas Compounds of Krypton(II) and Xenon(VI); [F5Xe(FKrF)AsF6] and [F5Xe(FKrF)2AsF6]
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2020-11-26 , DOI: 10.1002/anie.202014682 Matic Lozinšek 1, 2 , Hélène P. A. Mercier 1 , Gary J. Schrobilgen 1
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2020-11-26 , DOI: 10.1002/anie.202014682 Matic Lozinšek 1, 2 , Hélène P. A. Mercier 1 , Gary J. Schrobilgen 1
Affiliation
|
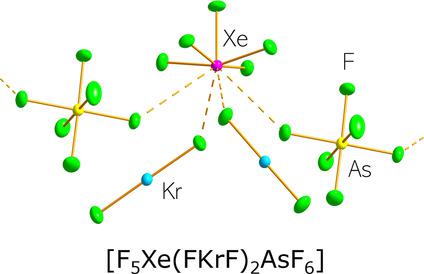
The coordination chemistry of KrF2 has been limited in contrast with that of XeF2, which exhibits a far richer coordination chemistry with main‐group and transition‐metal cations. In the present work, reactions of [XeF5][AsF6] with KrF2 in anhydrous HF solvent afforded [F5Xe(FKrF)AsF6] and [F5Xe(FKrF)2AsF6], the first mixed krypton/xenon compounds. X‐ray crystal structures and Raman spectra show the KrF2 ligands and [AsF6]− anions are F‐coordinated to the xenon atoms of the [XeF5]+ cations. Quantum‐chemical calculations are consistent with essentially noncovalent ligand−xenon bonds that may be described in terms of σ‐hole bonding. These complexes significantly extend the XeF2–KrF2 analogy and the limited chemistry of krypton by introducing a new class of coordination compound in which KrF2 functions as a ligand that coordinates to xenon(VI). The HF solvates, [F5Xe(FH)AsF6] and [F5Xe(FH)SbF6], are also characterized in this study and they provide rare examples of HF coordinated to xenon(VI).
中文翻译:

K(II)和氙(VI)的混合稀有气体化合物;[F5Xe(FKrF)AsF6]和[F5Xe(FKrF)2AsF6]
与XeF 2相比,KrF 2的配位化学受到了限制,而XeF 2的配位化学与主族和过渡金属阳离子的配合化学要丰富得多。在目前的工作中,[XeF 5 ] [AsF 6 ]与KrF 2在无水HF溶剂中的反应得到[F 5 Xe(FKrF)AsF 6 ]和[F 5 Xe(FKrF)2 AsF 6 ],第一种混合k /氙化合物。X射线晶体结构和拉曼光谱显示KrF 2配体和[AsF 6 ] -阴离子与[XeF 5 ]的氙原子F配位] +阳离子。量子化学计算与基本上非共价的配体-氙键相一致,可以用σ-孔键表示。这些配合物通过引入一类新的配位化合物来显着扩展了XeF 2 -KrF 2类比和and的有限化学,其中KrF 2充当配位至氙(VI)的配体。HF溶剂化物[F 5 Xe(FH)AsF 6 ]和[F 5 Xe(FH)SbF 6 ]在本研究中也得到了表征,它们提供了与氙(VI)配位的HF的罕见实例。
更新日期:2020-11-26
中文翻译:

K(II)和氙(VI)的混合稀有气体化合物;[F5Xe(FKrF)AsF6]和[F5Xe(FKrF)2AsF6]
与XeF 2相比,KrF 2的配位化学受到了限制,而XeF 2的配位化学与主族和过渡金属阳离子的配合化学要丰富得多。在目前的工作中,[XeF 5 ] [AsF 6 ]与KrF 2在无水HF溶剂中的反应得到[F 5 Xe(FKrF)AsF 6 ]和[F 5 Xe(FKrF)2 AsF 6 ],第一种混合k /氙化合物。X射线晶体结构和拉曼光谱显示KrF 2配体和[AsF 6 ] -阴离子与[XeF 5 ]的氙原子F配位] +阳离子。量子化学计算与基本上非共价的配体-氙键相一致,可以用σ-孔键表示。这些配合物通过引入一类新的配位化合物来显着扩展了XeF 2 -KrF 2类比和and的有限化学,其中KrF 2充当配位至氙(VI)的配体。HF溶剂化物[F 5 Xe(FH)AsF 6 ]和[F 5 Xe(FH)SbF 6 ]在本研究中也得到了表征,它们提供了与氙(VI)配位的HF的罕见实例。


























 京公网安备 11010802027423号
京公网安备 11010802027423号