Water Research ( IF 12.8 ) Pub Date : 2020-11-26 , DOI: 10.1016/j.watres.2020.116688 Changhui Wang , Zhanling Wang , Huacheng Xu , Leilei Bai , Cheng Liu , Helong Jiang , Peixin Cui
|
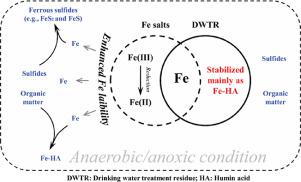
Fe-based materials used to adsorb P are commonly considered to be limited by the increased Fe lability, while Fe in drinking water treatment residue (DWTR) shows stable P adsorption abilities. Accordingly, this study aimed to gain insight into Fe lability in DWTR as compared to FeCl3 and Fe2(SO4)3 using Fe fractionation, EXAFS, and high-throughput sequencing technologies. The results showed that compared to Fe2(SO4)3 and FeCl3, Fe was relatively stable in the DWTR under the effects of organic matter, sulfides, and anaerobic conditions. Typically, the addition of FeCl3 and Fe2(SO4)3 enhanced Fe mobility in sediment and overlying water, promoting the formation of Fe-humin acid and ferrous sulfides (FeS and FeS2). However, the addition of DWTR, even at relatively high doses of Fe, has limited impact on Fe mobility. The addition remarkably increased oxidizable Fe in sediment (by approximately 63%), causing Fe to be dominated by oxidizable and residual fractions (like those in raw DWTR); EXAFS analysis also suggested that Fe-humin acid increased substantially with the addition of DWTR, becoming the main Fe species in sediment (with a relative abundance of 50.1%). Importantly, the Fe distributions were stable in sediment with DWTR added, which demonstrated that organic matter stabilized the Fe in the DWTR. Further analysis indicated that all materials promoted the enrichment of bacterial genera potentially related to Fe metabolism (e.g., Bacteroides, Dok59, and Methanosarcina). Fe2O3 in the FeCl3 and Fe2(SO4)3 groups and Fe-HA in the DWTR group were the key species affecting the microbial communities. Overall, the stabilizing effect of organic matter on Fe in DWTR could be used to develop Fe-based materials to enhance Fe stability for environmental remediation.
中文翻译:

有机物稳定饮用水处理残渣中的铁,对环境修复具有影响
通常认为,用于吸附P的铁基材料受Fe稳定性提高的限制,而饮用水处理残渣(DWTR)中的Fe显示出稳定的P吸附能力。因此,本研究旨在通过使用Fe分馏,EXAFS和高通量测序技术来深入了解DWTR中与FeCl 3和Fe 2(SO 4)3相比的Fe可靠性。结果表明,与Fe 2(SO 4)3和FeCl 3相比,在有机物,硫化物和厌氧条件下,DWTR中的Fe相对稳定。通常,添加FeCl 3和Fe 2(SO4)3增强了沉积物和上层水中的铁迁移率,促进了腐殖酸和硫化亚铁(FeS和FeS 2)。但是,即使在较高剂量的铁中添加DWTR,对铁迁移率的影响也有限。该添加显着增加了沉积物中的可氧化铁(约63%),从而使Fe被可氧化部分和残留部分(如原始DWTR中的那些)所支配。EXAFS分析还表明,随着DWTR的添加,腐殖酸铁含量大幅增加,成为沉积物中主要的Fe种类(相对丰度为50.1%)。重要的是,添加DWTR后,沉积物中的铁分布稳定,这表明有机物稳定了DWTR中的铁。进一步的分析表明,所有材料都促进了可能与铁代谢有关的细菌属的富集(例如,拟杆菌,Dok59和甲烷菌)。铁2FeCl 3和Fe 2(SO 4)3组中的O 3和DWTR组中的Fe-HA是影响微生物群落的关键物种。总体而言,DWTR中有机物对铁的稳定作用可用于开发铁基材料,以增强用于环境修复的铁稳定性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号