Journal of Molecular Biology ( IF 5.6 ) Pub Date : 2020-11-26 , DOI: 10.1016/j.jmb.2020.11.025 Elena Ostertag , Liujuan Zheng , Karina Broger , Thilo Stehle , Shu-Ming Li , Georg Zocher
|
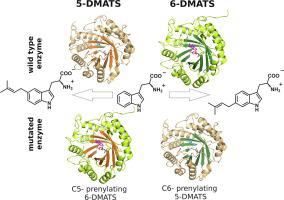
Prenylation is a process widely prevalent in primary and secondary metabolism, contributing to functionality and chemical diversity in natural systems. Due to their high regio- and chemoselectivities, prenyltransferases are also valuable tools for creation of new compounds by chemoenzymatic synthesis and synthetic biology. Over the last ten years, biochemical and structural investigations shed light on the mechanism and key residues that control the catalytic process, but to date crucial information on how certain prenyltransferases control regioselectivity and chemoselectivity is still lacking. Here, we advance a general understanding of the enzyme family by contributing the first structure of a tryptophan C5-prenyltransferase 5-DMATS. Additinally, the structure of a bacterial tryptophan C6-prenyltransferase 6-DMATS was solved. Analysis and comparison of both substrate-bound complexes led to the identification of key residues for catalysis. Next, site-directed mutagenesis was successfully implemented to not only modify the prenyl donor specificity but also to redirect the prenylation, thereby switching the regioselectivity of 6-DMATS to that of 5-DMATS. The general strategy of structure-guided protein engineering should be applicable to other related prenyltransferases, thus enabling the production of novel prenylated compounds.
中文翻译:

重编程底物和色氨酸异戊二烯基转移酶的催化滥交。
烯丙基化是在初级和次级代谢中广泛流行的过程,有助于天然系统的功能和化学多样性。由于异戊二烯基转移酶具有很高的区域选择性和化学选择性,因此它们也是通过化学酶促合成和合成生物学产生新化合物的有价值的工具。在过去的十年中,生物化学和结构研究揭示了控制催化过程的机理和关键残基,但迄今为止,仍然缺乏有关某些异戊烯基转移酶如何控制区域选择性和化学选择性的重要信息。在这里,我们通过贡献色氨酸C5-异戊二烯基转移酶5-DMATS的第一个结构,提高了对酶家族的一般理解。另外,解决了细菌色氨酸C6-异戊二烯基转移酶6-DMATS的结构。对两种底物结合的复合物的分析和比较导致了催化关键残基的鉴定。接下来,成功地进行了定点诱变,不仅修饰了异戊二烯基供体的特异性,而且使异戊二烯基重新定向,从而将6-DMATS的区域选择性转换为5-DMATS的区域选择性。结构指导的蛋白质工程的一般策略应适用于其他相关的异戊二烯基转移酶,从而能够生产新型的烯丙基化化合物。


























 京公网安备 11010802027423号
京公网安备 11010802027423号