当前位置:
X-MOL 学术
›
Atmos. Environ.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Theoretical investigation on the degradation of methyl vinyl ketone initiated by ·OH and ·Cl in the atmosphere and aqueous particles: Mechanism, kinetics, and environmental impact analysis
Atmospheric Environment ( IF 5 ) Pub Date : 2021-02-01 , DOI: 10.1016/j.atmosenv.2020.118107 Xueyu Wang , Lei Bao , Dandan Han , Yaoyao Wei , Maoxia He , Shiling Yuan
Atmospheric Environment ( IF 5 ) Pub Date : 2021-02-01 , DOI: 10.1016/j.atmosenv.2020.118107 Xueyu Wang , Lei Bao , Dandan Han , Yaoyao Wei , Maoxia He , Shiling Yuan
|
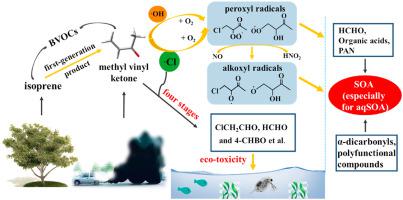
Abstract The absence of studies on the tropospheric reaction mechanism of unsaturated ketones increases the difficulty for the accurate assessment of their environmental fate. Herein, the gaseous and aqueous chemistry of methyl vinyl ketone (MVK) with ·Cl and ·OH, as well as the ensuing radical (e.g., chloroperoxy and hydroxyperoxy radicals) reactions are investigated from a theoretical perspective. A new pathway for the formation of alkoxyl radicals from peroxyl radicals is presumed. The reaction activity of alkoxyl radicals takes the order of β–scission > with O2 > with H2O > Russell mechanism. The aqueous environment shows the negative solvent effect on these two systems. The “polarization” of the proportion of the addition and abstraction reactions is intensified by the solvent effect. Although the rate constants kMVK-Cl > kMVK-OH > kMVK-O3, ·OH-induced MVK reaction is still the dominant sink of MVK. If the effect of ·Cl is ignored, the atmospheric lifetimes of MVK will be overestimated by 12% and even more. The detailed reaction mechanisms and product information for the reaction of ·Cl with MVK are calculated for the first time. Low volatility oxygenated products (such as organic acids, α-dicarbonyls, and some C4 multifunctional molecules) produced in the title reactions may be an important bridge between unsaturated ketone and aqSOA. An increased eco-toxicity can occur from MVK to its degradation products in the ·Cl-MVK system, highlighting the necessity in the research of LMW unsaturated hydrocarbon on environmental risk assessment.
中文翻译:

大气和水性颗粒中·OH和·Cl引发的甲基乙烯基酮降解的理论研究:机理、动力学和环境影响分析
摘要 由于缺乏对不饱和酮的对流层反应机制的研究,增加了对其环境归宿进行准确评估的难度。在此,从理论角度研究了甲基乙烯基酮 (MVK) 与·Cl 和·OH 的气态和水性化学以及随后的自由基(例如,氯过氧和羟基过氧自由基)反应。推测了从过氧自由基形成烷氧基自由基的新途径。烷氧基自由基的反应活性顺序为β-断裂>与O2>与H2O>罗素机制。水性环境显示出对这两个系统的负面溶剂影响。溶剂效应加剧了加成反应和脱除反应比例的“极化”。尽管速率常数 kMVK-Cl > kMVK-OH > kMVK-O3,·OH诱导的MVK反应仍然是MVK的主要汇。如果忽略·Cl的影响,MVK的大气寿命将被高估12%甚至更多。首次计算了·Cl与MVK反应的详细反应机理和产物信息。标题反应中产生的低挥发性含氧产物(如有机酸、α-二羰基化合物和一些 C4 多功能分子)可能是不饱和酮和 aqSOA 之间的重要桥梁。在·Cl-MVK体系中MVK对其降解产物的生态毒性增加,突出了LMW不饱和烃在环境风险评估中研究的必要性。MVK 的大气寿命将被高估 12% 甚至更多。首次计算了·Cl与MVK反应的详细反应机理和产物信息。标题反应中产生的低挥发性含氧产物(如有机酸、α-二羰基化合物和一些 C4 多功能分子)可能是不饱和酮和 aqSOA 之间的重要桥梁。在·Cl-MVK体系中MVK对其降解产物的生态毒性增加,突出了LMW不饱和烃在环境风险评估中研究的必要性。MVK 的大气寿命将被高估 12% 甚至更多。首次计算了·Cl与MVK反应的详细反应机理和产物信息。标题反应中产生的低挥发性含氧产物(如有机酸、α-二羰基化合物和一些 C4 多功能分子)可能是不饱和酮和 aqSOA 之间的重要桥梁。在·Cl-MVK体系中MVK对其降解产物的生态毒性增加,突出了LMW不饱和烃在环境风险评估中研究的必要性。标题反应中产生的 α-二羰基化合物和一些 C4 多功能分子)可能是不饱和酮和 aqSOA 之间的重要桥梁。在·Cl-MVK体系中MVK对其降解产物的生态毒性增加,突出了LMW不饱和烃在环境风险评估中研究的必要性。标题反应中产生的 α-二羰基化合物和一些 C4 多功能分子)可能是不饱和酮和 aqSOA 之间的重要桥梁。在·Cl-MVK体系中MVK对其降解产物的生态毒性增加,突出了LMW不饱和烃在环境风险评估中研究的必要性。
更新日期:2021-02-01
中文翻译:

大气和水性颗粒中·OH和·Cl引发的甲基乙烯基酮降解的理论研究:机理、动力学和环境影响分析
摘要 由于缺乏对不饱和酮的对流层反应机制的研究,增加了对其环境归宿进行准确评估的难度。在此,从理论角度研究了甲基乙烯基酮 (MVK) 与·Cl 和·OH 的气态和水性化学以及随后的自由基(例如,氯过氧和羟基过氧自由基)反应。推测了从过氧自由基形成烷氧基自由基的新途径。烷氧基自由基的反应活性顺序为β-断裂>与O2>与H2O>罗素机制。水性环境显示出对这两个系统的负面溶剂影响。溶剂效应加剧了加成反应和脱除反应比例的“极化”。尽管速率常数 kMVK-Cl > kMVK-OH > kMVK-O3,·OH诱导的MVK反应仍然是MVK的主要汇。如果忽略·Cl的影响,MVK的大气寿命将被高估12%甚至更多。首次计算了·Cl与MVK反应的详细反应机理和产物信息。标题反应中产生的低挥发性含氧产物(如有机酸、α-二羰基化合物和一些 C4 多功能分子)可能是不饱和酮和 aqSOA 之间的重要桥梁。在·Cl-MVK体系中MVK对其降解产物的生态毒性增加,突出了LMW不饱和烃在环境风险评估中研究的必要性。MVK 的大气寿命将被高估 12% 甚至更多。首次计算了·Cl与MVK反应的详细反应机理和产物信息。标题反应中产生的低挥发性含氧产物(如有机酸、α-二羰基化合物和一些 C4 多功能分子)可能是不饱和酮和 aqSOA 之间的重要桥梁。在·Cl-MVK体系中MVK对其降解产物的生态毒性增加,突出了LMW不饱和烃在环境风险评估中研究的必要性。MVK 的大气寿命将被高估 12% 甚至更多。首次计算了·Cl与MVK反应的详细反应机理和产物信息。标题反应中产生的低挥发性含氧产物(如有机酸、α-二羰基化合物和一些 C4 多功能分子)可能是不饱和酮和 aqSOA 之间的重要桥梁。在·Cl-MVK体系中MVK对其降解产物的生态毒性增加,突出了LMW不饱和烃在环境风险评估中研究的必要性。标题反应中产生的 α-二羰基化合物和一些 C4 多功能分子)可能是不饱和酮和 aqSOA 之间的重要桥梁。在·Cl-MVK体系中MVK对其降解产物的生态毒性增加,突出了LMW不饱和烃在环境风险评估中研究的必要性。标题反应中产生的 α-二羰基化合物和一些 C4 多功能分子)可能是不饱和酮和 aqSOA 之间的重要桥梁。在·Cl-MVK体系中MVK对其降解产物的生态毒性增加,突出了LMW不饱和烃在环境风险评估中研究的必要性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号