当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Solvent selection for biphasic extraction of 5-hydroxymethylfurfural via multiscale modeling and experiments
Green Chemistry ( IF 9.8 ) Pub Date : 2020-11-19 , DOI: 10.1039/d0gc03251d Zhaoxing Wang 1, 2, 3, 4, 5 , Souryadeep Bhattacharyya 1, 2, 3, 4, 5 , Dionisios G. Vlachos 1, 2, 3, 4, 5
Green Chemistry ( IF 9.8 ) Pub Date : 2020-11-19 , DOI: 10.1039/d0gc03251d Zhaoxing Wang 1, 2, 3, 4, 5 , Souryadeep Bhattacharyya 1, 2, 3, 4, 5 , Dionisios G. Vlachos 1, 2, 3, 4, 5
Affiliation
|
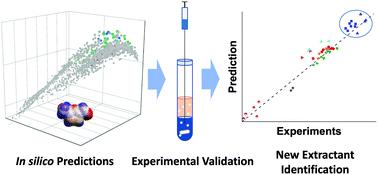
We introduce a comprehensive conceptual framework for selecting solvents for reactive extraction in biphasic organic-water systems and demonstrate it for the separation of HMF (5-hydroxylmethylfurfural), a platform chemical produced in the acid-catalyzed dehydration of hexoses. We first perform in silico screening of ∼2500 solvents, from the ADFCRS-2018 database using the ADF COSMO-RS implementation, and classification, based on the solvent partition coefficient. We then determine experimentally the partition coefficients for HMF, fructose, and products of HMF rehydration (levulinic acid (LA), and formic acid (FA)), the mutual water-organic solvent solubilities, and the separation factors in >50 select solvents spanning multiple homologous series at room temperature and a typical reaction temperature with in situ sampling. We find that COSMO-RS is excellent for screening purposes (typical error in most cases within a factor of ∼2). Increased temperatures lead to significant reduction in partitioning, and room temperature measurements are clearly inadequate for solvent selection. Upon down selecting classes of solvents based on separation performance, we perform experimental thermal stability and reaction compatibility studies of a small set of solvents at relevant reactive-extraction temperatures. We discover that many substituted phenols exhibit an order-of-magnitude increase in partitioning compared to conventional solvents due chiefly to hydrogen bond interactions and show the necessary stability but retain a significant fraction of water and LA, factors that need to be considered in technoeconomic analysis. In contrast, anilines, aldehydes, and acids are good to excellent regarding separation but incompatible with this specific reaction medium. This multifaceted framework can be extended to other biomass-derived products and processes.
中文翻译:

多尺度建模和实验双相萃取5-羟甲基糠醛的溶剂选择
我们介绍了用于选择在双相有机水系统中进行反应萃取的溶剂的全面概念框架,并展示了其用于分离HMF(5-羟甲基糠醛)的方法,HMF是在酸催化的己糖脱水中生成的一种平台化学品。我们首先使用ADF COSMO-RS实现方法从ADFCRS-2018数据库中对约2500种溶剂进行计算机筛选,并基于溶剂分配系数进行分类。然后,我们通过实验确定HMF,果糖和HMF补液产物(乙酰丙酸(LA)和甲酸(FA))的分配系数,水-有机溶剂的互溶性以及在> 50的精选溶剂中的分离系数在室温和典型反应温度下具有多个同源序列原位采样。我们发现COSMO-RS对于筛选目的是极好的(大多数情况下的典型误差在2左右)。升高的温度会导致分配显着减少,并且室温测量显然不足以选择溶剂。根据分离性能向下选择溶剂类别后,我们在相关的反应萃取温度下对少量溶剂进行了实验热稳定性和反应相容性研究。我们发现,与常规溶剂相比,许多取代的苯酚在分配方面表现出数量级的增加,这主要是由于氢键之间的相互作用,并显示出必要的稳定性,但保留了大量的水和LA,这是技术经济分析中需要考虑的因素。相比之下,苯胺,醛,酸和酸在分离方面优良至极好,但与这种特定的反应介质不相容。这个多方面的框架可以扩展到其他生物质衍生的产品和过程。
更新日期:2020-11-25
中文翻译:

多尺度建模和实验双相萃取5-羟甲基糠醛的溶剂选择
我们介绍了用于选择在双相有机水系统中进行反应萃取的溶剂的全面概念框架,并展示了其用于分离HMF(5-羟甲基糠醛)的方法,HMF是在酸催化的己糖脱水中生成的一种平台化学品。我们首先使用ADF COSMO-RS实现方法从ADFCRS-2018数据库中对约2500种溶剂进行计算机筛选,并基于溶剂分配系数进行分类。然后,我们通过实验确定HMF,果糖和HMF补液产物(乙酰丙酸(LA)和甲酸(FA))的分配系数,水-有机溶剂的互溶性以及在> 50的精选溶剂中的分离系数在室温和典型反应温度下具有多个同源序列原位采样。我们发现COSMO-RS对于筛选目的是极好的(大多数情况下的典型误差在2左右)。升高的温度会导致分配显着减少,并且室温测量显然不足以选择溶剂。根据分离性能向下选择溶剂类别后,我们在相关的反应萃取温度下对少量溶剂进行了实验热稳定性和反应相容性研究。我们发现,与常规溶剂相比,许多取代的苯酚在分配方面表现出数量级的增加,这主要是由于氢键之间的相互作用,并显示出必要的稳定性,但保留了大量的水和LA,这是技术经济分析中需要考虑的因素。相比之下,苯胺,醛,酸和酸在分离方面优良至极好,但与这种特定的反应介质不相容。这个多方面的框架可以扩展到其他生物质衍生的产品和过程。


























 京公网安备 11010802027423号
京公网安备 11010802027423号