当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A Greener Alternative for the Synthesis of Methyl Pentenone Using Reusable Cation Exchange Resin
Industrial & Engineering Chemistry Research ( IF 4.2 ) Pub Date : 2020-11-25 , DOI: 10.1021/acs.iecr.0c03864 Sumit Kamal 1 , Sanjay Mahajani 1
Industrial & Engineering Chemistry Research ( IF 4.2 ) Pub Date : 2020-11-25 , DOI: 10.1021/acs.iecr.0c03864 Sumit Kamal 1 , Sanjay Mahajani 1
Affiliation
|
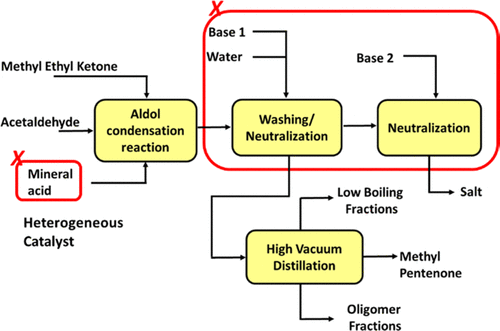
Synthesis of methyl pentenone (MPO) using the aldol condensation reaction of methyl ethyl ketone (MEK) and acetaldehyde (AcH) in the presence of mineral acid is a commercially practiced process. The reaction is associated with the formation of a number of side products. We propose an environment-friendly, commercially available heterogeneous cation exchange resin (e.g., Ambelryst-15) catalyst for this reaction and examine its efficacy under optimized conditions. Amberlyst-15 offers better yield as compared to its homogeneous counterpart. The selectivity of the desired product was found to be increasing with the conversion of acetaldehyde, which prompted us to explore the detailed chemistry of the process. The major side products include the oligomers of acetaldehyde of different molecular weights formed by various self- and repeated aldol condensation reactions. The reaction kinetics is studied by performing experiments in batch mode in the presence of Ambelryst-15 (average diameter of 600 μm) at 600 RPM to avoid effects of external mass transfer resistances. The effect of different parameters such as particle size, stirrer speed, temperature, composition, and catalyst loading on the reaction performance is investigated. A detailed kinetic model is proposed based on the Langmuir–Hinshelwood–Hougen–Watson (LHHW) mechanism, which explains the progress of main and side reactions satisfactorily. The proposed route that uses cation exchange resin catalyst holds a potential to make the process more compact and environment-friendly.
中文翻译:

使用可重复使用的阳离子交换树脂合成甲基戊烯酮的绿色替代方法
使用甲基乙基酮(MEK)和乙醛(AcH)的醛醇缩合反应在无机酸存在下合成甲基戊烯酮(MPO)是一种工业实践方法。该反应与许多副产物的形成有关。我们提出了一种环境友好的可商购的非均相阳离子交换树脂(例如Ambelryst-15)催化剂,用于该反应,并在优化条件下检查其功效。与同类产品相比,Amberlyst-15的产量更高。发现所需产物的选择性随着乙醛的转化而增加,这促使我们探索该方法的详细化学过程。主要副产物包括通过各种自重和重复的醇醛缩合反应形成的不同分子量的乙醛低聚物。通过在Ambelryst-15(平均直径为600μm)存在下以600 RPM进行分批模式实验来研究反应动力学,以避免外部传质阻力的影响。研究了不同参数(例如粒度,搅拌速度,温度,组成和催化剂负载量)对反应性能的影响。基于Langmuir-Hinshelwood-Hougen-Watson(LHHW)机理,提出了详细的动力学模型,该模型令人满意地解释了主反应和副反应的进展。所提出的使用阳离子交换树脂催化剂的途径具有使该方法更紧凑和环境友好的潜力。通过在Ambelryst-15(平均直径为600μm)存在下以600 RPM进行分批模式实验来研究反应动力学,以避免外部传质阻力的影响。研究了不同参数(例如粒度,搅拌速度,温度,组成和催化剂负载量)对反应性能的影响。基于Langmuir-Hinshelwood-Hougen-Watson(LHHW)机理,提出了详细的动力学模型,该模型令人满意地解释了主反应和副反应的进展。所提出的使用阳离子交换树脂催化剂的途径具有使该方法更紧凑和环境友好的潜力。通过在Ambelryst-15(平均直径为600μm)存在下以600 RPM进行分批模式实验来研究反应动力学,以避免外部传质阻力的影响。研究了不同参数(例如粒度,搅拌速度,温度,组成和催化剂负载量)对反应性能的影响。基于Langmuir-Hinshelwood-Hougen-Watson(LHHW)机理,提出了详细的动力学模型,该模型令人满意地解释了主反应和副反应的进展。所提出的使用阳离子交换树脂催化剂的途径具有使该方法更紧凑和环境友好的潜力。
更新日期:2020-12-09
中文翻译:

使用可重复使用的阳离子交换树脂合成甲基戊烯酮的绿色替代方法
使用甲基乙基酮(MEK)和乙醛(AcH)的醛醇缩合反应在无机酸存在下合成甲基戊烯酮(MPO)是一种工业实践方法。该反应与许多副产物的形成有关。我们提出了一种环境友好的可商购的非均相阳离子交换树脂(例如Ambelryst-15)催化剂,用于该反应,并在优化条件下检查其功效。与同类产品相比,Amberlyst-15的产量更高。发现所需产物的选择性随着乙醛的转化而增加,这促使我们探索该方法的详细化学过程。主要副产物包括通过各种自重和重复的醇醛缩合反应形成的不同分子量的乙醛低聚物。通过在Ambelryst-15(平均直径为600μm)存在下以600 RPM进行分批模式实验来研究反应动力学,以避免外部传质阻力的影响。研究了不同参数(例如粒度,搅拌速度,温度,组成和催化剂负载量)对反应性能的影响。基于Langmuir-Hinshelwood-Hougen-Watson(LHHW)机理,提出了详细的动力学模型,该模型令人满意地解释了主反应和副反应的进展。所提出的使用阳离子交换树脂催化剂的途径具有使该方法更紧凑和环境友好的潜力。通过在Ambelryst-15(平均直径为600μm)存在下以600 RPM进行分批模式实验来研究反应动力学,以避免外部传质阻力的影响。研究了不同参数(例如粒度,搅拌速度,温度,组成和催化剂负载量)对反应性能的影响。基于Langmuir-Hinshelwood-Hougen-Watson(LHHW)机理,提出了详细的动力学模型,该模型令人满意地解释了主反应和副反应的进展。所提出的使用阳离子交换树脂催化剂的途径具有使该方法更紧凑和环境友好的潜力。通过在Ambelryst-15(平均直径为600μm)存在下以600 RPM进行分批模式实验来研究反应动力学,以避免外部传质阻力的影响。研究了不同参数(例如粒度,搅拌速度,温度,组成和催化剂负载量)对反应性能的影响。基于Langmuir-Hinshelwood-Hougen-Watson(LHHW)机理,提出了详细的动力学模型,该模型令人满意地解释了主反应和副反应的进展。所提出的使用阳离子交换树脂催化剂的途径具有使该方法更紧凑和环境友好的潜力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号