当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Alternative Kinetic Model of the Iodide–Iodate Reaction for Its Use in Micromixing Investigations
Industrial & Engineering Chemistry Research ( IF 4.2 ) Pub Date : 2020-11-25 , DOI: 10.1021/acs.iecr.0c04901 Arturo N. Manzano Martı́nez 1 , A. Sander Haase 2 , Melissa Assirelli 2 , John van der Schaaf 1
Industrial & Engineering Chemistry Research ( IF 4.2 ) Pub Date : 2020-11-25 , DOI: 10.1021/acs.iecr.0c04901 Arturo N. Manzano Martı́nez 1 , A. Sander Haase 2 , Melissa Assirelli 2 , John van der Schaaf 1
Affiliation
|
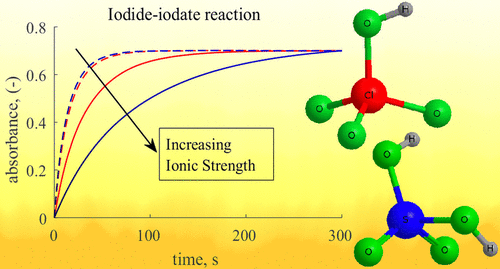
The Villermaux–Dushman method, one of the most extensively used test reaction systems for micromixing characterization, has been widely criticized for years due to uncertainties regarding the incomplete dissociation of sulfuric acid and the proposed kinetic study by Guichardon et al. In this work, a renewed study of the kinetics of the iodide–iodate reaction is presented, using perchloric acid to avoid issues concerning incomplete acid dissociation. The experimental results are in good agreement with the fifth-order rate law for the iodide–iodate reaction. The reaction rate coefficient strongly depends on the ionic strength and can be modeled with a Davies-like equation. When implemented in the incorporation model, the kinetic model presented in this study can be used to estimate micromixing times that are in line with the theoretical engulfment time. This is observed in two different reactors with low and high intensities of mixing: an unbaffled stirred vessel and a rotor–stator spinning disk reactor. The results from the latter are also compared with the second Bourne reaction, giving very similar micromixing times. The kinetic model presented in this study in combination with strong monoprotic acids is suggested as an alternative to characterize micromixing behavior.
中文翻译:

碘化物-碘酸盐反应的替代动力学模型用于微混合研究
维勒莫-杜什曼方法是用于微混合表征的最广泛使用的测试反应系统之一,由于对硫酸不完全解离的不确定性以及Guichardon等人提出的动力学研究,多年来一直受到广泛批评。在这项工作中,提出了对碘化物-碘酸盐反应动力学的新研究,使用高氯酸来避免有关酸解离不完全的问题。实验结果与碘化物-碘酸盐反应的五阶速率定律非常吻合。反应速率系数很大程度上取决于离子强度,可以用Davies-like方程建模。在公司合并模型中实施时,在这项研究中提出的动力学模型可以用来估计与理论吞噬时间一致的微混合时间。这在两种混合反应强度高和低的反应器中观察到:无挡板的搅拌容器和转子-定子旋转盘反应器。后者的结果也与第二个Bourne反应进行了比较,给出了非常相似的微混合时间。建议在这项研究中提出的动力学模型与强单质子酸相结合,作为表征微混合行为的替代方法。
更新日期:2020-12-09
中文翻译:

碘化物-碘酸盐反应的替代动力学模型用于微混合研究
维勒莫-杜什曼方法是用于微混合表征的最广泛使用的测试反应系统之一,由于对硫酸不完全解离的不确定性以及Guichardon等人提出的动力学研究,多年来一直受到广泛批评。在这项工作中,提出了对碘化物-碘酸盐反应动力学的新研究,使用高氯酸来避免有关酸解离不完全的问题。实验结果与碘化物-碘酸盐反应的五阶速率定律非常吻合。反应速率系数很大程度上取决于离子强度,可以用Davies-like方程建模。在公司合并模型中实施时,在这项研究中提出的动力学模型可以用来估计与理论吞噬时间一致的微混合时间。这在两种混合反应强度高和低的反应器中观察到:无挡板的搅拌容器和转子-定子旋转盘反应器。后者的结果也与第二个Bourne反应进行了比较,给出了非常相似的微混合时间。建议在这项研究中提出的动力学模型与强单质子酸相结合,作为表征微混合行为的替代方法。


























 京公网安备 11010802027423号
京公网安备 11010802027423号