Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Photomodulating Carbon Dots for Spatiotemporal Suppression of Alzheimer’s β-Amyloid Aggregation
ACS Nano ( IF 17.1 ) Pub Date : 2020-11-25 , DOI: 10.1021/acsnano.0c06078 You Jung Chung 1 , Chang Heon Lee 1 , Jinyeong Lim 2 , Jinhyeong Jang 1 , Hyuno Kang 3 , Chan Beum Park 1
ACS Nano ( IF 17.1 ) Pub Date : 2020-11-25 , DOI: 10.1021/acsnano.0c06078 You Jung Chung 1 , Chang Heon Lee 1 , Jinyeong Lim 2 , Jinhyeong Jang 1 , Hyuno Kang 3 , Chan Beum Park 1
Affiliation
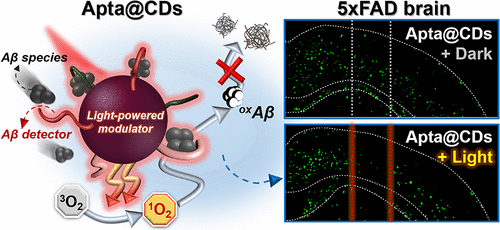
|
Extracellular deposition of β-amyloid (Aβ) peptide aggregates is a major characteristic of Alzheimer’s disease (AD) brain. Because Aβ peptide aggregates aggravate neuropathy and cognitive impairment for AD patients, numerous efforts have been devoted to suppressing Aβ self-assembly as a prospective AD treatment option. Here, we report Aβ-targeting, red-light-responsive carbon dots (CDs), and their therapeutic functions as a light-powered nanomodulator to spatiotemporally suppress toxic Aβ aggregation both in vitro and in vivo. Our aptamer-functionalized carbon dots (Apta@CDs) showed strong targeting ability toward Aβ42 species. Moreover, red LED irradiation induced Apta@CDs to irreversibly denature Aβ peptides, impeding the formation of β-sheet-rich Aβ aggregates and attenuating Aβ-associated cytotoxicity. Consequently, Apta@CDs-mediated photomodualtion modality achieved effective suppression of Aβ aggregation in vivo, which significantly reduced the Aβ burden at the targeted sites in the brain of 5xFAD mice by ∼40% and ∼25% according to imaging and ELISA analyses, respectively. Our work demonstrates the therapeutic potential of photomodulating CDs for light-driven suppression against Aβ self-assembly and related neurotoxicity.
中文翻译:

光调制碳点的时空抑制阿尔茨海默氏症的β淀粉样蛋白聚集。
β-淀粉样蛋白(Aβ)肽聚集体的细胞外沉积是阿尔茨海默病(AD)脑的主要特征。由于Aβ肽聚集会加剧AD患者的神经病变和认知功能障碍,因此人们已经做出了许多努力来抑制Aβ自组装作为一种潜在的AD治疗选择。在这里,我们报道了靶向Aβ的红光响应碳点(CDs),以及它们作为光动力纳米调节剂的治疗功能,可在体外和体内时空抑制有毒的Aβ聚集。我们的适体功能化碳点(Apta @ CD)对Aβ42表现出强大的靶向能力种类。此外,红色LED辐射诱导Apta @ CDs不可逆地使Aβ肽变性,从而阻碍了富含β-折叠的Aβ聚集体的形成并减弱了Aβ相关的细胞毒性。因此,Apta @ CDs介导的光模态方式在体内可有效抑制Aβ聚集,根据成像和ELISA分析,其分别将5xFAD小鼠大脑目标部位的Aβ负担降低了约40%和〜25%。 。我们的工作证明了光调节CD对光驱动抑制Aβ自组装和相关神经毒性的治疗潜力。
更新日期:2020-11-25
中文翻译:

光调制碳点的时空抑制阿尔茨海默氏症的β淀粉样蛋白聚集。
β-淀粉样蛋白(Aβ)肽聚集体的细胞外沉积是阿尔茨海默病(AD)脑的主要特征。由于Aβ肽聚集会加剧AD患者的神经病变和认知功能障碍,因此人们已经做出了许多努力来抑制Aβ自组装作为一种潜在的AD治疗选择。在这里,我们报道了靶向Aβ的红光响应碳点(CDs),以及它们作为光动力纳米调节剂的治疗功能,可在体外和体内时空抑制有毒的Aβ聚集。我们的适体功能化碳点(Apta @ CD)对Aβ42表现出强大的靶向能力种类。此外,红色LED辐射诱导Apta @ CDs不可逆地使Aβ肽变性,从而阻碍了富含β-折叠的Aβ聚集体的形成并减弱了Aβ相关的细胞毒性。因此,Apta @ CDs介导的光模态方式在体内可有效抑制Aβ聚集,根据成像和ELISA分析,其分别将5xFAD小鼠大脑目标部位的Aβ负担降低了约40%和〜25%。 。我们的工作证明了光调节CD对光驱动抑制Aβ自组装和相关神经毒性的治疗潜力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号