当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis and Degradation of Polyphosphate: Isotope Effects in Enzyme- and Bacteria-Catalyzed Reactions
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2020-11-25 , DOI: 10.1021/acsearthspacechem.0c00230 Yuge Bai 1, 2 , Lisa Stout 1 , Gulcin Unal-Tosun 1 , Jiying Li 1, 3 , Deb Jaisi 1
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2020-11-25 , DOI: 10.1021/acsearthspacechem.0c00230 Yuge Bai 1, 2 , Lisa Stout 1 , Gulcin Unal-Tosun 1 , Jiying Li 1, 3 , Deb Jaisi 1
Affiliation
|
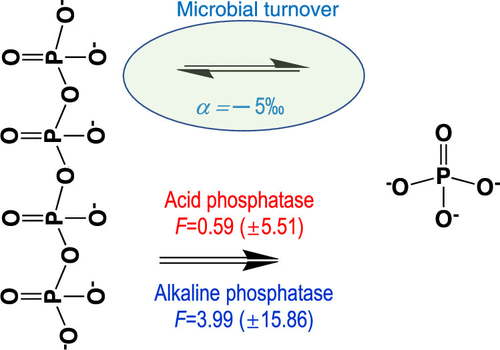
Polyphosphate (poly-P) is a chain of phosphate moieties linked through high-energy phosphoanhydride bond, and it plays an important role in regulatory functions in prokaryotic cells. Isotope effects of bacterial synthesis and degradation of poly-P may provide important insights into the roles of poly-P in environmental phosphorus (P) cycling. In this research, we investigated the enzymatic degradation of poly-P by cell-free enzyme solutions and bacterial cells (Escherichia coli JM103 and Pseudomonas putida KT2440) and analyzed phosphate oxygen isotope ratios (δ18OP), enzyme activity, and dynamics of orthophosphate (Pi) and poly-P. Cell-free enzyme reaction results show that both acid and alkaline phosphatase enzymes are capable of catalyzing poly-P degradation, albeit with different efficiencies of >70 and <18%, respectively. Isotope fractionation factors during enzymatic degradation of poly-P were slightly positive [+0.59 (±5.51) to +3.99 (±15.86)‰] for acid and alkaline phosphatase, respectively, which is rather uncommon in phosphatase enzyme-catalyzed degradation of many other organic P compounds. Poly-P is synthesized during the exponential growth phase of E. coli, causing an isotope fractionation of −5.65 (±1.02)‰ leading to the enrichment of isotope in the residual Pi in the growth media. Degradation of poly-P occurred at the late stationary phase, with lighter isotope values of Pi in the growth media. These results imply that the specific isotopes, fractionation, and P dynamics during poly-P synthesis and degradation could serve as proxies to interpret poly-P dynamics in the environment.
中文翻译:

多磷酸盐的合成和降解:酶和细菌催化反应中的同位素效应
聚磷酸盐(poly-P)是通过高能磷酸酐键连接的磷酸部分链,在原核细胞的调节功能中起重要作用。细菌合成和poly-P降解的同位素效应可能为poly-P在环境磷(P)循环中的作用提供重要见解。在本研究中,我们通过无细胞的酶溶液和细菌细胞(研究聚磷的酶促降解的大肠杆菌JM103和恶臭假单胞菌KT2440)并分析磷酸盐氧同位素比率(δ 18 ö P),酶的活性,和动力学正磷酸盐(P i)和poly-P。无细胞酶反应结果表明,酸性和碱性磷酸酶都能够催化多聚P降解,尽管效率分别> 70和<18%。酸和碱性磷酸酶分别在多聚酶的酶降解过程中的同位素分馏因子略为阳性[+0.59(±5.51)至+3.99(±15.86)‰],这在磷酸酶催化的许多其他酶的降解中并不常见有机P化合物。Poly-P在大肠杆菌的指数生长期合成,导致同位素分馏为-5.65(±1.02)‰,导致同位素在生长培养基中的残留P i中富集。poly-P的降解发生在固定后期,P的同位素值更轻我在成长型媒体中。这些结果暗示在poly-P合成和降解过程中特定的同位素,分馏和P动力学可以作为解释环境中poly-P动力学的代理。
更新日期:2020-12-17
中文翻译:

多磷酸盐的合成和降解:酶和细菌催化反应中的同位素效应
聚磷酸盐(poly-P)是通过高能磷酸酐键连接的磷酸部分链,在原核细胞的调节功能中起重要作用。细菌合成和poly-P降解的同位素效应可能为poly-P在环境磷(P)循环中的作用提供重要见解。在本研究中,我们通过无细胞的酶溶液和细菌细胞(研究聚磷的酶促降解的大肠杆菌JM103和恶臭假单胞菌KT2440)并分析磷酸盐氧同位素比率(δ 18 ö P),酶的活性,和动力学正磷酸盐(P i)和poly-P。无细胞酶反应结果表明,酸性和碱性磷酸酶都能够催化多聚P降解,尽管效率分别> 70和<18%。酸和碱性磷酸酶分别在多聚酶的酶降解过程中的同位素分馏因子略为阳性[+0.59(±5.51)至+3.99(±15.86)‰],这在磷酸酶催化的许多其他酶的降解中并不常见有机P化合物。Poly-P在大肠杆菌的指数生长期合成,导致同位素分馏为-5.65(±1.02)‰,导致同位素在生长培养基中的残留P i中富集。poly-P的降解发生在固定后期,P的同位素值更轻我在成长型媒体中。这些结果暗示在poly-P合成和降解过程中特定的同位素,分馏和P动力学可以作为解释环境中poly-P动力学的代理。


























 京公网安备 11010802027423号
京公网安备 11010802027423号