当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
General Synthesis of Secondary Alkylamines by Reductive Alkylation of Nitriles by Aldehydes and Ketones
Chemistry - A European Journal ( IF 4.3 ) Pub Date : 2020-11-25 , DOI: 10.1002/chem.202004755 Timon Schönauer 1 , Sabrina L. J. Thomä 2 , Leah Kaiser 1 , Mirijam Zobel 2 , Rhett Kempe 1
Chemistry - A European Journal ( IF 4.3 ) Pub Date : 2020-11-25 , DOI: 10.1002/chem.202004755 Timon Schönauer 1 , Sabrina L. J. Thomä 2 , Leah Kaiser 1 , Mirijam Zobel 2 , Rhett Kempe 1
Affiliation
|
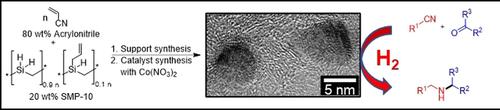
The development of C−N bond formation reactions is highly desirable due to their importance in biology and chemistry. Recent progress in 3d metal catalysis is indicative of unique selectivity patterns that may permit solving challenges of chemical synthesis. We report here on a catalytic C−N bond formation reaction—the reductive alkylation of nitriles. Aldehydes or ketones and nitriles, all abundantly available and low‐cost starting materials, undergo a reductive coupling to form secondary alkylamines and inexpensive hydrogen is used as the reducing agent. The reaction has a very broad scope and many functional groups, including hydrogenation‐sensitive examples, are tolerated. We developed a novel cobalt catalyst, which is nanostructured, reusable, and easy to handle. The key seems the earth‐abundant metal in combination with a porous support material, N‐doped SiC, synthesized from acrylonitrile and a commercially available polycarbosilane.
中文翻译:

通过醛和酮对腈的还原性烷基化反应一般合成仲烷基胺
由于它们在生物学和化学中的重要性,因此非常需要开发CN键形成反应。3d金属催化的最新进展表明,独特的选择性模式可以解决化学合成难题。我们在这里报告催化性C-N键形成反应-腈的还原烷基化。醛,酮和腈都是可得的廉价原料,它们经过还原偶合形成仲烷基胺,廉价的氢用作还原剂。该反应的范围非常广泛,并且可以耐受许多官能团,包括对氢化敏感的实例。我们开发了一种新型的钴催化剂,该催化剂具有纳米结构,可重复使用且易于处理。
更新日期:2021-01-21
中文翻译:

通过醛和酮对腈的还原性烷基化反应一般合成仲烷基胺
由于它们在生物学和化学中的重要性,因此非常需要开发CN键形成反应。3d金属催化的最新进展表明,独特的选择性模式可以解决化学合成难题。我们在这里报告催化性C-N键形成反应-腈的还原烷基化。醛,酮和腈都是可得的廉价原料,它们经过还原偶合形成仲烷基胺,廉价的氢用作还原剂。该反应的范围非常广泛,并且可以耐受许多官能团,包括对氢化敏感的实例。我们开发了一种新型的钴催化剂,该催化剂具有纳米结构,可重复使用且易于处理。


























 京公网安备 11010802027423号
京公网安备 11010802027423号