当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Acceptor Engineering for Optimized ROS Generation Facilitates Reprogramming Macrophages to M1 Phenotype in Photodynamic Immunotherapy
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2020-11-25 , DOI: 10.1002/anie.202013228 Guang Yang 1 , Jen-Shyang Ni 1 , Yaxi Li 1 , Menglei Zha 1 , Yao Tu 1 , Kai Li 1
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2020-11-25 , DOI: 10.1002/anie.202013228 Guang Yang 1 , Jen-Shyang Ni 1 , Yaxi Li 1 , Menglei Zha 1 , Yao Tu 1 , Kai Li 1
Affiliation
|
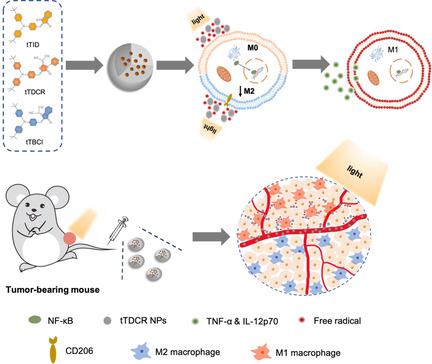
Reprogramming tumor‐associated macrophages to an antitumor M1 phenotype by photodynamic therapy is a promising strategy to overcome the immunosuppression of tumor microenvironment for boosted immunotherapy. However, it remains unclear how the reactive oxygen species (ROS) generated from type I and II mechanisms, relate to the macrophage polarization efficacy. Herein, we design and synthesize three donor–acceptor structured photosensitizers with varied ROS‐generating efficiencies. Surprisingly, we discovered that the extracellular ROS generated from type I mechanism are mainly responsible for reprogramming the macrophages from a pro‐tumor type (M2) to an anti‐tumor state (M1). In vivo experiments prove that the photosensitizer can trigger photodynamic immunotherapy for effective suppression of the tumor growth, while the therapeutic outcome is abolished with depleted macrophages. Overall, our strategy highlights the designing guideline of macrophage‐activatable photosensitizers.
中文翻译:

优化的ROS生成的受体工程有助于在光动力免疫治疗中将巨噬细胞重编程为M1表型。
通过光动力疗法将与肿瘤相关的巨噬细胞重编程为抗肿瘤M1表型是一种有前景的策略,可以克服对肿瘤微环境的免疫抑制,从而增强免疫疗法。然而,目前尚不清楚从I型和II型机理产生的活性氧(ROS)与巨噬细胞极化功效之间的关系。本文中,我们设计和合成了三种具有不同ROS产生效率的供体-受体结构的光敏剂。出乎意料的是,我们发现由I型机制产生的细胞外ROS主要负责将巨噬细胞从前肿瘤类型(M2)重编程为抗肿瘤状态(M1)。体内实验证明,光敏剂可以触发光动力免疫疗法,以有效抑制肿瘤的生长,耗尽的巨噬细胞可消除治疗结果。总体而言,我们的策略突出了可激活巨噬细胞的光敏剂的设计指南。
更新日期:2020-11-25
中文翻译:

优化的ROS生成的受体工程有助于在光动力免疫治疗中将巨噬细胞重编程为M1表型。
通过光动力疗法将与肿瘤相关的巨噬细胞重编程为抗肿瘤M1表型是一种有前景的策略,可以克服对肿瘤微环境的免疫抑制,从而增强免疫疗法。然而,目前尚不清楚从I型和II型机理产生的活性氧(ROS)与巨噬细胞极化功效之间的关系。本文中,我们设计和合成了三种具有不同ROS产生效率的供体-受体结构的光敏剂。出乎意料的是,我们发现由I型机制产生的细胞外ROS主要负责将巨噬细胞从前肿瘤类型(M2)重编程为抗肿瘤状态(M1)。体内实验证明,光敏剂可以触发光动力免疫疗法,以有效抑制肿瘤的生长,耗尽的巨噬细胞可消除治疗结果。总体而言,我们的策略突出了可激活巨噬细胞的光敏剂的设计指南。



























 京公网安备 11010802027423号
京公网安备 11010802027423号