当前位置:
X-MOL 学术
›
Nano Today
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
In situ pepsin-assisted needle assembly of magnetic-graphitic-nanocapsules for enhanced gastric retention and mucus penetration
Nano Today ( IF 17.4 ) Pub Date : 2020-11-25 , DOI: 10.1016/j.nantod.2020.101032 Xinqi Cai , Yiting Xu , Lina Zhao , Jiamei Xu , Shengkai Li , Chaoqi Wen , Xin Xia , Qian Dong , Xiaoxiao Hu , Xiaofeng Wang , Long Chen , Zhuo Chen , Weihong Tan
Nano Today ( IF 17.4 ) Pub Date : 2020-11-25 , DOI: 10.1016/j.nantod.2020.101032 Xinqi Cai , Yiting Xu , Lina Zhao , Jiamei Xu , Shengkai Li , Chaoqi Wen , Xin Xia , Qian Dong , Xiaoxiao Hu , Xiaofeng Wang , Long Chen , Zhuo Chen , Weihong Tan
|
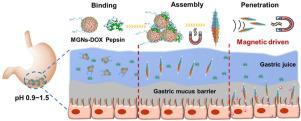
Gastric environment is an extreme pH and enzyme-rich condition, which together with gastric mucus barrier and short retention time of oral medicine militate against effective oral drug delivery to lesion areas of gastric diseases. Assembly, especially shape-control assembly of magnetic nanoparticles potentially helps the site-selective drug molecule delivery. However, the harsh gastric condition, and cumbersome and strict requirements make the in situ reversible assembly remain challenging. Herein, synergistically pepsin-bridged and magnetic field-mediated morphological control of magnetic-graphitic-nanocapsules (MGNs) needle assembly (MNA) is developed in stomach for prolonged gastric retention and enhanced mucus penetration. The MNAs with good biocompatibility, large magnetic moments and unique needle-shape are formed with the help of pepsin, demonstrating superior magnetic-driven capability to overcome gastric mucus barrier. Molecular dynamics simulations reveal binding modes between MGN and amino acid residues on either side of the pepsin, indicating pepsin acts as “bridge” allowing sufficient contact among MGNs and further facilitates the needle assembly. Moreover, anticancer drug doxorubicin (DOX)-loaded MNAs demonstrate superior targeted cancer cell killing ability and boosted DOX penetration in a mouse model, which promises good bioavailability of drug molecules. The versatile MNA-based platform offers a robust strategy for effective oral drug delivery and site-selective therapy of gastric diseases.
中文翻译:

原位胃蛋白酶辅助磁性石墨纳米胶囊针组装,增强胃潴留和粘液渗透
胃内环境pH值极端、酶丰富,加上胃粘液屏障和口服药物保留时间短,不利于口服药物有效输送至胃病病变部位。磁性纳米粒子的组装,特别是形状控制组装可能有助于位点选择性药物分子递送。然而,恶劣的胃条件以及繁琐和严格的要求使得原位可逆组装仍然具有挑战性。在此,在胃中开发了胃蛋白酶桥联和磁场介导的磁性石墨纳米胶囊(MGN)针组件(MNA)的协同形态控制,以延长胃滞留时间并增强粘液渗透性。在胃蛋白酶的帮助下形成具有良好生物相容性、大磁矩和独特针状形状的MNA,表现出优异的磁驱动能力来克服胃粘液屏障。分子动力学模拟揭示了 MGN 与胃蛋白酶两侧氨基酸残基之间的结合模式,表明胃蛋白酶充当“桥梁”,允许 MGN 之间充分接触,并进一步促进针组装。此外,负载抗癌药物阿霉素(DOX)的MNA在小鼠模型中表现出优异的靶向癌细胞杀伤能力并增强了DOX渗透,这保证了药物分子良好的生物利用度。基于 MNA 的多功能平台为有效的口服药物递送和胃部疾病的位点选择性治疗提供了强有力的策略。
更新日期:2020-11-25
中文翻译:

原位胃蛋白酶辅助磁性石墨纳米胶囊针组装,增强胃潴留和粘液渗透
胃内环境pH值极端、酶丰富,加上胃粘液屏障和口服药物保留时间短,不利于口服药物有效输送至胃病病变部位。磁性纳米粒子的组装,特别是形状控制组装可能有助于位点选择性药物分子递送。然而,恶劣的胃条件以及繁琐和严格的要求使得原位可逆组装仍然具有挑战性。在此,在胃中开发了胃蛋白酶桥联和磁场介导的磁性石墨纳米胶囊(MGN)针组件(MNA)的协同形态控制,以延长胃滞留时间并增强粘液渗透性。在胃蛋白酶的帮助下形成具有良好生物相容性、大磁矩和独特针状形状的MNA,表现出优异的磁驱动能力来克服胃粘液屏障。分子动力学模拟揭示了 MGN 与胃蛋白酶两侧氨基酸残基之间的结合模式,表明胃蛋白酶充当“桥梁”,允许 MGN 之间充分接触,并进一步促进针组装。此外,负载抗癌药物阿霉素(DOX)的MNA在小鼠模型中表现出优异的靶向癌细胞杀伤能力并增强了DOX渗透,这保证了药物分子良好的生物利用度。基于 MNA 的多功能平台为有效的口服药物递送和胃部疾病的位点选择性治疗提供了强有力的策略。


























 京公网安备 11010802027423号
京公网安备 11010802027423号