Applied Catalysis B: Environment and Energy ( IF 22.1 ) Pub Date : 2020-11-25 , DOI: 10.1016/j.apcatb.2020.119729 Sang-Yeon Lee , Ik-Sun Kim , Hyun-Seok Cho , Chang-Hee Kim , Yong-Kul Lee
|
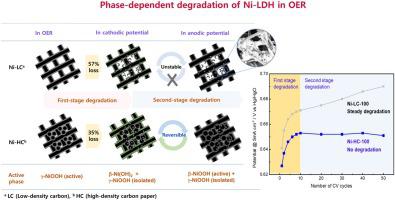
The activation and degradation mechanism of Ni-LDH catalysts electrodeposited on low and high-density carbon papers for the oxygen evolution reaction (OER) in alkaline media was investigated using in situ X-ray absorption near-edge structure (XANES) spectroscopy coupled with CV cycles in a potential range of 0–0.9 V (vs Hg/HgO), which allowed proposing two-stage degradation mechanisms with respect to cyclic voltammetry (CV) cycles. The electrodeposited α-Ni(OH)2 is firstly transformed to γ-NiOOH as an active phase in OER. In the reducible potential region, however, γ-NiOOH was partially reduced to β-Ni(OH)2, isolating the rest, which is the first stage of degradation. In the following anodic potential region, β-Ni(OH)2 is readily converted to β-NiOOH, which is mostly reversible, but only a small portion of β-NiOOH is overcharged to unstable γ-NiOOH, being responsible for the second stage degradation. It was noted that Ni(OH)2 catalysts electrodeposited on a low-density carbon paper (Ni-LC) underwent a severe degradation in the first stage, losing at least 56.9 % current density at 0.65 V (vs Hg/HgO), followed by a steady degradation in the second stage, while the use of a high-density carbon substrate (Ni−HC) effectively improved redox stability, maintaining a minimal loss of overpotential less than 5%, particularly with the second stage degradation being negligible even under potential changes, providing an important insight into designing durable and active Ni-LDH catalysts for the OER.
中文翻译:

解决碱性氧析出反应(OER)中电沉积Ni(OH)2催化剂的电位依赖性降解:原位XANES研究
利用原位X射线吸收近边缘结构(XANES)和CV技术研究了在低密度和高密度碳纸上电沉积Ni-LDH催化剂在碱性介质中的氧释放反应(OER)的活化和降解机理。在0-0.9 V(vs Hg / HgO)的电位范围内进行循环,这允许就循环伏安(CV)循环提出两阶段降解机理。电沉积的α-Ni(OH)2首先在OER中转变为γ-NiOOH作为活性相。但是,在可还原电位区域,γ-NiOOH被部分还原为β-Ni(OH)2,其余部分被隔离,这是降解的第一阶段。在以下阳极电势区域中,β-Ni(OH)2β-NiOOH容易转化为大部分可逆的β-NiOOH,但只有一小部分β-NiOOH被过度充电至不稳定的γ-NiOOH,这是第二阶段降解的原因。值得注意的是,电沉积在低密度碳纸(Ni-LC)上的Ni(OH)2催化剂在第一阶段经历了严重降解,在0.65 V(vs Hg / HgO)下损失了至少56.9%的电流密度,随后通过第二阶段的稳定降解,同时使用高密度碳基质(Ni-HC)有效改善了氧化还原稳定性,将过电势的最小损失保持在5%以下,尤其是第二阶段的降解可以忽略不计潜在的变化,为设计用于OER的耐用和活性Ni-LDH催化剂提供了重要的见识。



























 京公网安备 11010802027423号
京公网安备 11010802027423号