当前位置:
X-MOL 学术
›
Fuel Process. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Catalytic hydrogenation and etherification of 5-Hydroxymethylfurfural into 2-(alkoxymethyl)-5-methylfuran and 2,5-bis(alkoxymethyl)furan as potential biofuel additives
Fuel Processing Technology ( IF 7.5 ) Pub Date : 2021-03-01 , DOI: 10.1016/j.fuproc.2020.106672 Islam Elsayed , Michael A. Jackson , El Barbary Hassan
Fuel Processing Technology ( IF 7.5 ) Pub Date : 2021-03-01 , DOI: 10.1016/j.fuproc.2020.106672 Islam Elsayed , Michael A. Jackson , El Barbary Hassan
|
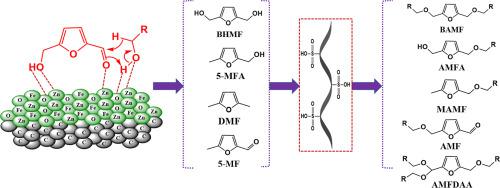
Abstract In this study, 5-hydroxymethylfurfural (HMF) was converted into several biofuel additives such as alkoxymethylfurans (AMFs) and 2,5-bis(alkoxymethyl)furans (BAMFs) through two-step sequential hydrogenation and etherification reactions. In the first step, zinc‑iron magnetic nanocatalyst supported on activated carbon (ZnO-Fe3O4/AC) was prepared for the selective hydrogenation of HMF into BHMF and 5-MFA via Meerwein-Ponndorf-Verley (MPV) reaction in three different hydrogen donor alcohols (ethanol, 1-propanol, and 1-butanol). The important physical properties of the catalyst such as crystallinity, chemical composition, morphology, reduction behavior, and surface area were studied by using several analytical techniques. The effect of hydrogenation parameters such as catalyst concentration, temperature, and time on the selectivity of (BHMF and 5-MFA), and HMF conversion were studied. The best hydrogenation results were obtained with 0.2 mmole HMF and 100 mg of catalyst at 200 °C for 12 h. In the second step, three commercial Bronsted acid catalysts were used to convert the hydrogenated products into alkoxymethylfurans (AMFs) and 2,5-bis(alkoxymethyl)furans (BAMFs). At the optimum etherification conditions (65 °C and 10 h), a spectrum of mono-, di-, and tri- ether compounds were obtained. The hydrogenation catalyst (ZnO-Fe3O4/AC) was recycled and used for five times without a remarkable reduction in its catalytic activity.
中文翻译:

5-羟甲基糠醛催化氢化和醚化成 2-(烷氧基甲基)-5-甲基呋喃和 2,5-双(烷氧基甲基)呋喃作为潜在的生物燃料添加剂
摘要 本研究通过两步顺序加氢和醚化反应将 5-羟甲基糠醛 (HMF) 转化为烷氧基甲基呋喃 (AMF) 和 2,5-双(烷氧基甲基) 呋喃 (BAMF) 等多种生物燃料添加剂。第一步,制备活性炭负载的锌铁磁性纳米催化剂(ZnO-Fe3O4/AC),通过 Meerwein-Ponndorf-Verley(MPV)反应在三种不同的氢供体中将 HMF 选择性氢化为 BHMF 和 5-MFA醇类(乙醇、1-丙醇和 1-丁醇)。通过使用多种分析技术研究了催化剂的重要物理性质,例如结晶度、化学组成、形态、还原行为和表面积。加氢参数的影响,如催化剂浓度、温度、研究了(BHMF 和 5-MFA)的选择性和时间对 HMF 转化率的影响。使用 0.2 mmol HMF 和 100 mg 催化剂在 200 °C 下 12 小时获得最佳氢化结果。在第二步中,使用三种商业布朗斯台德酸催化剂将氢化产物转化为烷氧基甲基呋喃 (AMF) 和 2,5-双(烷氧基甲基)呋喃 (BAMF)。在最佳醚化条件(65 °C 和 10 小时)下,获得了一系列单醚、二醚和三醚化合物。加氢催化剂(ZnO-Fe3O4/AC)循环使用5次,催化活性没有明显降低。使用三种商业布朗斯台德酸催化剂将氢化产物转化为烷氧基甲基呋喃 (AMF) 和 2,5-双(烷氧基甲基)呋喃 (BAMF)。在最佳醚化条件(65 °C 和 10 小时)下,获得了一系列单醚、二醚和三醚化合物。加氢催化剂(ZnO-Fe3O4/AC)循环使用5次,催化活性没有明显降低。使用三种商业布朗斯台德酸催化剂将氢化产物转化为烷氧基甲基呋喃 (AMF) 和 2,5-双(烷氧基甲基)呋喃 (BAMF)。在最佳醚化条件(65 °C 和 10 小时)下,获得了一系列单醚、二醚和三醚化合物。加氢催化剂(ZnO-Fe3O4/AC)循环使用5次,催化活性没有明显降低。
更新日期:2021-03-01
中文翻译:

5-羟甲基糠醛催化氢化和醚化成 2-(烷氧基甲基)-5-甲基呋喃和 2,5-双(烷氧基甲基)呋喃作为潜在的生物燃料添加剂
摘要 本研究通过两步顺序加氢和醚化反应将 5-羟甲基糠醛 (HMF) 转化为烷氧基甲基呋喃 (AMF) 和 2,5-双(烷氧基甲基) 呋喃 (BAMF) 等多种生物燃料添加剂。第一步,制备活性炭负载的锌铁磁性纳米催化剂(ZnO-Fe3O4/AC),通过 Meerwein-Ponndorf-Verley(MPV)反应在三种不同的氢供体中将 HMF 选择性氢化为 BHMF 和 5-MFA醇类(乙醇、1-丙醇和 1-丁醇)。通过使用多种分析技术研究了催化剂的重要物理性质,例如结晶度、化学组成、形态、还原行为和表面积。加氢参数的影响,如催化剂浓度、温度、研究了(BHMF 和 5-MFA)的选择性和时间对 HMF 转化率的影响。使用 0.2 mmol HMF 和 100 mg 催化剂在 200 °C 下 12 小时获得最佳氢化结果。在第二步中,使用三种商业布朗斯台德酸催化剂将氢化产物转化为烷氧基甲基呋喃 (AMF) 和 2,5-双(烷氧基甲基)呋喃 (BAMF)。在最佳醚化条件(65 °C 和 10 小时)下,获得了一系列单醚、二醚和三醚化合物。加氢催化剂(ZnO-Fe3O4/AC)循环使用5次,催化活性没有明显降低。使用三种商业布朗斯台德酸催化剂将氢化产物转化为烷氧基甲基呋喃 (AMF) 和 2,5-双(烷氧基甲基)呋喃 (BAMF)。在最佳醚化条件(65 °C 和 10 小时)下,获得了一系列单醚、二醚和三醚化合物。加氢催化剂(ZnO-Fe3O4/AC)循环使用5次,催化活性没有明显降低。使用三种商业布朗斯台德酸催化剂将氢化产物转化为烷氧基甲基呋喃 (AMF) 和 2,5-双(烷氧基甲基)呋喃 (BAMF)。在最佳醚化条件(65 °C 和 10 小时)下,获得了一系列单醚、二醚和三醚化合物。加氢催化剂(ZnO-Fe3O4/AC)循环使用5次,催化活性没有明显降低。


























 京公网安备 11010802027423号
京公网安备 11010802027423号