当前位置:
X-MOL 学术
›
Colloids Surf. A Physicochem. Eng. Aspects
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The effect of CTAB, a cationic surfactant, on the adsorption ability of the boron-doped diamond electrode: Application for voltammetric sensing of Bisphenol A and Hydroquinone in water samples
Colloids and Surfaces A: Physicochemical and Engineering Aspects ( IF 5.2 ) Pub Date : 2021-02-01 , DOI: 10.1016/j.colsurfa.2020.125916 Saadi Ali Hoshyar , Hemn A.H. Barzani , Yavuz Yardım , Zühre Şentürk
Colloids and Surfaces A: Physicochemical and Engineering Aspects ( IF 5.2 ) Pub Date : 2021-02-01 , DOI: 10.1016/j.colsurfa.2020.125916 Saadi Ali Hoshyar , Hemn A.H. Barzani , Yavuz Yardım , Zühre Şentürk
|
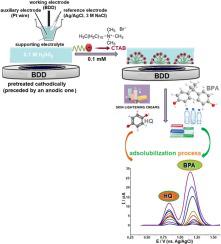
Abstract The present study introduces for the first time the effect of the cationic surfactant, cetyltrimethylammonium bromide (CTAB) on the adsorption capability of the boron-doped diamond (BDD) electrode for the sensing of environmental pollutants bisphenol A (BPA) and hydroquinone (HQ). Cathodic pretreatment of the electrode surface after an anodic pretreatment exhibited a best electrochemical response for these two phenolic compounds. The electrochemical characteristics of BPA and HQ were first sufficiently documented by cyclic voltammetry in aqueous solutions without the addition of surfactant over a shorter and longer potential range. Square-wave voltammetry was employed for a detailed study depending on the operational conditions (e.g., pH and nature of supporting electrolyte, concentration of CTAB, accumulation variables, instrumental parameters, etc.). The sensitivity of the voltammetric measurements for BPA and HQ was increased when CTAB was present in strong acidic media (more effective for BPA) due to its interaction with the neutral forms of the selected compounds on the hydrophobic surface of BDD electrode. Besides, a significant increase in the detecting sensitivity of BPA in CTAB-containing solutions could be obtained after applying an accumulation process. Using square-wave stripping mode (after 30 s accumulation at open-circuit condition) in 0.1 M H2SO4 containing 1 × 10-4 M CTAB, the limits of detection were found to be 0.05 µg/mL (2.19 × 10-7 M), and 0.23 µg/mL (2.09 × 10-6 M) for the simultaneous determination of BPA and HQ, respectively. In this way, modification-free BDD electrode could introduce an alternative to chemically modified electrodes for the analysis of environmental samples.
中文翻译:

CTAB(一种阳离子表面活性剂)对掺硼金刚石电极吸附能力的影响:在水样中双酚 A 和对苯二酚的伏安检测中的应用
摘要 本研究首次介绍了阳离子表面活性剂十六烷基三甲基溴化铵 (CTAB) 对硼掺杂金刚石 (BDD) 电极吸附环境污染物双酚 A (BPA) 和氢醌 (HQ) 吸附能力的影响。 )。阳极预处理后电极表面的阴极预处理对这两种酚类化合物表现出最好的电化学响应。BPA 和 HQ 的电化学特性首先在水溶液中通过循环伏安法得到充分证明,而无需在更短和更长的电位范围内添加表面活性剂。根据操作条件(例如,支持电解质的 pH 值和性质、CTAB 浓度、积累变量、仪器参数等)。当 CTAB 存在于强酸性介质中(对 BPA 更有效)时,BPA 和 HQ 伏安测量的灵敏度增加,因为它与 BDD 电极疏水表面上所选化合物的中性形式相互作用。此外,在应用累积过程后,可以显着提高含 CTAB 溶液中 BPA 的检测灵敏度。在含有 1 × 10-4 M CTAB 的 0.1 M H2SO4 中使用方波剥离模式(在开路条件下累积 30 秒后),发现检测限为 0.05 µg/mL (2.19 × 10-7 M)和 0.23 µg/mL (2.09 × 10-6 M) 分别用于同时测定 BPA 和 HQ。这样,
更新日期:2021-02-01
中文翻译:

CTAB(一种阳离子表面活性剂)对掺硼金刚石电极吸附能力的影响:在水样中双酚 A 和对苯二酚的伏安检测中的应用
摘要 本研究首次介绍了阳离子表面活性剂十六烷基三甲基溴化铵 (CTAB) 对硼掺杂金刚石 (BDD) 电极吸附环境污染物双酚 A (BPA) 和氢醌 (HQ) 吸附能力的影响。 )。阳极预处理后电极表面的阴极预处理对这两种酚类化合物表现出最好的电化学响应。BPA 和 HQ 的电化学特性首先在水溶液中通过循环伏安法得到充分证明,而无需在更短和更长的电位范围内添加表面活性剂。根据操作条件(例如,支持电解质的 pH 值和性质、CTAB 浓度、积累变量、仪器参数等)。当 CTAB 存在于强酸性介质中(对 BPA 更有效)时,BPA 和 HQ 伏安测量的灵敏度增加,因为它与 BDD 电极疏水表面上所选化合物的中性形式相互作用。此外,在应用累积过程后,可以显着提高含 CTAB 溶液中 BPA 的检测灵敏度。在含有 1 × 10-4 M CTAB 的 0.1 M H2SO4 中使用方波剥离模式(在开路条件下累积 30 秒后),发现检测限为 0.05 µg/mL (2.19 × 10-7 M)和 0.23 µg/mL (2.09 × 10-6 M) 分别用于同时测定 BPA 和 HQ。这样,



























 京公网安备 11010802027423号
京公网安备 11010802027423号