Chemical Physics Letters ( IF 2.8 ) Pub Date : 2020-11-24 , DOI: 10.1016/j.cplett.2020.138207 Younes Valadbeigi , Jean-François Gal
|
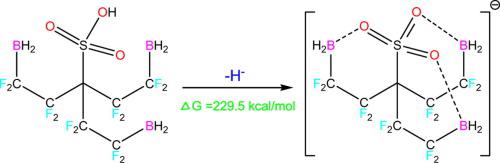
New organometallic superacids of sulfonic acid derivatives were designed on the basis of acidity enhancement by internal bonding with an electron-pair acceptor group -BX2 (X = H, F, Cl, Br). The acidity enhancement in the gas phase was assessed using the B3LYP/6-311++G(d,p) method. Two classes of superacids were devised. The first series was based on a cyclopentadiene ring carrying a -SO2(CH2)3BX2 or -SO(OH)(CH2)3BX2 group, and the second series was designed by substituting the three C-H of CH3SO3H by three –(CR2)3BX2 groups (R = H, F). An organometallic hyperacid of relatively small size with ΔacidG of 229.5 kcal mol-1 was obtained.
中文翻译:

有机金属超强酸和高强酸:通过与强电子对受体基团-BX 2的内部键合提高酸度
在通过与电子对受体基团-BX 2(X = H,F,Cl,Br)进行内部键合提高酸度的基础上,设计了磺酸衍生物的新型有机金属超酸。使用B3LYP / 6-311 ++ G(d,p)方法评估气相中的酸度增强。设计了两类超酸。第一个系列基于带有-SO 2(CH 2)3 BX 2或-SO(OH)(CH 2)3 BX 2基团的环戊二烯环,第二个系列是通过取代CH 3的三个CH设计的SO 3 H乘以3 –(CR 2)3 BX2组(R = H,F)。获得具有229.5kcal mol -1的Δ酸G的相对较小尺寸的有机金属高酸。



























 京公网安备 11010802027423号
京公网安备 11010802027423号