当前位置:
X-MOL 学术
›
J. Electroanal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electrochemical immunosensor based on metal ions functionalized CNSs@Au NPs nanocomposites as signal amplifier for simultaneous detection of triple tumor markers
Journal of Electroanalytical Chemistry ( IF 4.5 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.jelechem.2020.114882 Lihua Li , Yan Wei , Shengpeng Zhang , Xishan Chen , Taili Shao , Dexiang Feng
Journal of Electroanalytical Chemistry ( IF 4.5 ) Pub Date : 2021-01-01 , DOI: 10.1016/j.jelechem.2020.114882 Lihua Li , Yan Wei , Shengpeng Zhang , Xishan Chen , Taili Shao , Dexiang Feng
|
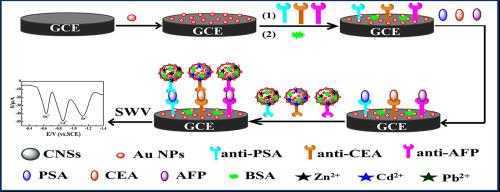
Abstract A multiple immunosensor was developed for simultaneous detection of prostate specific antigen (PSA), carcinoembryonic antigen (CEA) and α-fetoprotein (AFP). In this sensing platform, metal ions functionalized gold nanoparticles‑carbon nanospheres (CNSs@Au NPs) nanocomposites were firstly synthesized not only as an efficient immunoprobe but also as signal amplifier. Herein, CNSs@Au NPs with large specific surface area and excellent biocompatibility provided a good vehicle to load much more second antibodies. Metal ions (Pb2+、Cd2+、Zn2+) were fixed the surface of nanocomposites via strong binding capacity with amino-groups of second antibodies to form a signal amplifying system. Meanwhile, immunosensor was prepared by depositing Au NPs and coating primary antibodies on the glassy carbon electrode. In the existence of target analytes and immunoprobes, the immunosensor could simultaneously respond to multiple analyte targets and three distinguishable signal peaks of square wave voltammetry (SWV) were obtained. Under appropriate experimental conditions, the proposed immunosensor exhibited wider measurement linear ranges for three markers (PSA, 0.01 to 100 ng mL−1, CEA and AFP, 0.01 to 80 ng mL−1) with a low detection limits of PSA (3.6 pg mL−1), CEA (3.0 pg mL−1) and AFP (2.6 pg mL−1, S/N = 3). Finally, the as-prepared immunosensor was assessed with human serum samples. Compared with the reference method, no obvious difference was observed, indicating our immunosensor had a potential application in early clinical diagnosis of tumor markers.
中文翻译:

基于金属离子功能化CNSs@Au NPs纳米复合材料的电化学免疫传感器作为信号放大器同时检测三重肿瘤标志物
摘要 开发了一种多免疫传感器,用于同时检测前列腺特异性抗原(PSA)、癌胚抗原(CEA)和甲胎蛋白(AFP)。在这个传感平台上,金属离子功能化的金纳米粒子-碳纳米球(CNSs@Au NPs)纳米复合材料首先被合成,不仅可以作为有效的免疫探针,还可以作为信号放大器。在此,具有大比表面积和优异生物相容性的 CNS@Au NPs 为加载更多二抗提供了良好的载体。金属离子(Pb2+、Cd2+、Zn2+)通过与二抗氨基的强结合能力固定在纳米复合材料的表面,形成信号放大系统。同时,通过在玻碳电极上沉积Au NPs并涂覆一抗来制备免疫传感器。在目标分析物和免疫探针存在的情况下,免疫传感器可以同时响应多个分析物目标,并获得方波伏安法(SWV)的三个可区分信号峰。在适当的实验条件下,所提出的免疫传感器对三种标志物(PSA,0.01 至 100 ng mL-1,CEA 和 AFP,0.01 至 80 ng mL-1)表现出更宽的测量线性范围,PSA 检测限低(3.6 pg mL-1) -1)、CEA (3.0 pg mL-1) 和 AFP (2.6 pg mL-1,S/N = 3)。最后,用人血清样品评估所制备的免疫传感器。与参考方法相比,未观察到明显差异,表明我们的免疫传感器在肿瘤标志物的早期临床诊断中具有潜在的应用价值。免疫传感器可以同时响应多个分析物目标,并获得了方波伏安法 (SWV) 的三个可区分信号峰。在适当的实验条件下,所提出的免疫传感器对三种标志物(PSA,0.01 至 100 ng mL-1,CEA 和 AFP,0.01 至 80 ng mL-1)表现出更宽的测量线性范围,PSA 检测限低(3.6 pg mL-1) -1)、CEA (3.0 pg mL-1) 和 AFP (2.6 pg mL-1,S/N = 3)。最后,用人血清样品评估所制备的免疫传感器。与参考方法相比,未观察到明显差异,表明我们的免疫传感器在肿瘤标志物的早期临床诊断中具有潜在的应用价值。免疫传感器可以同时响应多个分析物目标,并获得了方波伏安法 (SWV) 的三个可区分信号峰。在适当的实验条件下,所提出的免疫传感器对三种标志物(PSA,0.01 至 100 ng mL-1,CEA 和 AFP,0.01 至 80 ng mL-1)表现出更宽的测量线性范围,PSA 检测限低(3.6 pg mL-1) -1)、CEA (3.0 pg mL-1) 和 AFP (2.6 pg mL-1,S/N = 3)。最后,用人血清样品评估所制备的免疫传感器。与参考方法相比,未观察到明显差异,表明我们的免疫传感器在肿瘤标志物的早期临床诊断中具有潜在的应用价值。所提出的免疫传感器对三种标志物(PSA,0.01 至 100 ng mL-1,CEA 和 AFP,0.01 至 80 ng mL-1)表现出更宽的测量线性范围,PSA(3.6 pg mL-1),CEA 的检测限较低(3.0 pg mL-1) 和 AFP (2.6 pg mL-1,S/N = 3)。最后,用人血清样品评估所制备的免疫传感器。与参考方法相比,未观察到明显差异,表明我们的免疫传感器在肿瘤标志物的早期临床诊断中具有潜在的应用价值。所提出的免疫传感器对三种标志物(PSA,0.01 至 100 ng mL-1,CEA 和 AFP,0.01 至 80 ng mL-1)表现出更宽的测量线性范围,PSA(3.6 pg mL-1),CEA 的检测限较低(3.0 pg mL-1) 和 AFP (2.6 pg mL-1,S/N = 3)。最后,用人血清样品评估所制备的免疫传感器。与参考方法相比,未观察到明显差异,表明我们的免疫传感器在肿瘤标志物的早期临床诊断中具有潜在的应用价值。
更新日期:2021-01-01
中文翻译:

基于金属离子功能化CNSs@Au NPs纳米复合材料的电化学免疫传感器作为信号放大器同时检测三重肿瘤标志物
摘要 开发了一种多免疫传感器,用于同时检测前列腺特异性抗原(PSA)、癌胚抗原(CEA)和甲胎蛋白(AFP)。在这个传感平台上,金属离子功能化的金纳米粒子-碳纳米球(CNSs@Au NPs)纳米复合材料首先被合成,不仅可以作为有效的免疫探针,还可以作为信号放大器。在此,具有大比表面积和优异生物相容性的 CNS@Au NPs 为加载更多二抗提供了良好的载体。金属离子(Pb2+、Cd2+、Zn2+)通过与二抗氨基的强结合能力固定在纳米复合材料的表面,形成信号放大系统。同时,通过在玻碳电极上沉积Au NPs并涂覆一抗来制备免疫传感器。在目标分析物和免疫探针存在的情况下,免疫传感器可以同时响应多个分析物目标,并获得方波伏安法(SWV)的三个可区分信号峰。在适当的实验条件下,所提出的免疫传感器对三种标志物(PSA,0.01 至 100 ng mL-1,CEA 和 AFP,0.01 至 80 ng mL-1)表现出更宽的测量线性范围,PSA 检测限低(3.6 pg mL-1) -1)、CEA (3.0 pg mL-1) 和 AFP (2.6 pg mL-1,S/N = 3)。最后,用人血清样品评估所制备的免疫传感器。与参考方法相比,未观察到明显差异,表明我们的免疫传感器在肿瘤标志物的早期临床诊断中具有潜在的应用价值。免疫传感器可以同时响应多个分析物目标,并获得了方波伏安法 (SWV) 的三个可区分信号峰。在适当的实验条件下,所提出的免疫传感器对三种标志物(PSA,0.01 至 100 ng mL-1,CEA 和 AFP,0.01 至 80 ng mL-1)表现出更宽的测量线性范围,PSA 检测限低(3.6 pg mL-1) -1)、CEA (3.0 pg mL-1) 和 AFP (2.6 pg mL-1,S/N = 3)。最后,用人血清样品评估所制备的免疫传感器。与参考方法相比,未观察到明显差异,表明我们的免疫传感器在肿瘤标志物的早期临床诊断中具有潜在的应用价值。免疫传感器可以同时响应多个分析物目标,并获得了方波伏安法 (SWV) 的三个可区分信号峰。在适当的实验条件下,所提出的免疫传感器对三种标志物(PSA,0.01 至 100 ng mL-1,CEA 和 AFP,0.01 至 80 ng mL-1)表现出更宽的测量线性范围,PSA 检测限低(3.6 pg mL-1) -1)、CEA (3.0 pg mL-1) 和 AFP (2.6 pg mL-1,S/N = 3)。最后,用人血清样品评估所制备的免疫传感器。与参考方法相比,未观察到明显差异,表明我们的免疫传感器在肿瘤标志物的早期临床诊断中具有潜在的应用价值。所提出的免疫传感器对三种标志物(PSA,0.01 至 100 ng mL-1,CEA 和 AFP,0.01 至 80 ng mL-1)表现出更宽的测量线性范围,PSA(3.6 pg mL-1),CEA 的检测限较低(3.0 pg mL-1) 和 AFP (2.6 pg mL-1,S/N = 3)。最后,用人血清样品评估所制备的免疫传感器。与参考方法相比,未观察到明显差异,表明我们的免疫传感器在肿瘤标志物的早期临床诊断中具有潜在的应用价值。所提出的免疫传感器对三种标志物(PSA,0.01 至 100 ng mL-1,CEA 和 AFP,0.01 至 80 ng mL-1)表现出更宽的测量线性范围,PSA(3.6 pg mL-1),CEA 的检测限较低(3.0 pg mL-1) 和 AFP (2.6 pg mL-1,S/N = 3)。最后,用人血清样品评估所制备的免疫传感器。与参考方法相比,未观察到明显差异,表明我们的免疫传感器在肿瘤标志物的早期临床诊断中具有潜在的应用价值。


























 京公网安备 11010802027423号
京公网安备 11010802027423号