当前位置:
X-MOL 学术
›
CrystEngComm
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Increasing the structural boundary of quasiracemate formation: 4-substituted naphthylamides
CrystEngComm ( IF 3.1 ) Pub Date : 2020-11-17 , DOI: 10.1039/d0ce01331e Drew E. Craddock 1, 2, 3, 4 , McKenzie J. Parks 1, 2, 3, 4 , Lauren A. Taylor 1, 2, 3, 4 , Benjamin L. Wagner 1, 4, 5, 6 , Michael Ruf 4, 7, 8 , Kraig A. Wheeler 1, 2, 3, 4
CrystEngComm ( IF 3.1 ) Pub Date : 2020-11-17 , DOI: 10.1039/d0ce01331e Drew E. Craddock 1, 2, 3, 4 , McKenzie J. Parks 1, 2, 3, 4 , Lauren A. Taylor 1, 2, 3, 4 , Benjamin L. Wagner 1, 4, 5, 6 , Michael Ruf 4, 7, 8 , Kraig A. Wheeler 1, 2, 3, 4
Affiliation
|
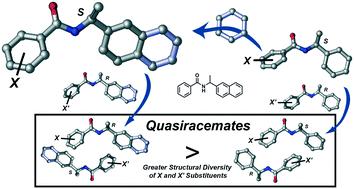
Quasiracemates – materials consisting of pairs of near enantiomers – form crystalline motifs that mimic the inversion relationships observed for their racemic counterparts. Recent investigations from our group explored a family of chiral (N-benzoyl)methylbenzylamines to understand the structural boundary of cocrystallization. This investigation extends these earlier studies to include naphthylamide quasiracemates, where the molecular framework is ∼20% larger by volume than the previous diarylamides. A family of naphthylamides was prepared where the pendant functional group differs incrementally in size (i.e., H to C6H5) to give 55 possible unique pairs of racemic and quasiracemic combinations. Data collected from these materials using X-ray crystallography, thermal analysis methods and lattice energy calculations offer important insight into how a spatially larger naphthylamide molecular framework promotes greater structural variance of substituents during the pairwise assembly of quasienantiomers.
中文翻译:

增加准外消旋物形成的结构边界:4-取代的萘甲酰胺
准外消旋物(由接近对映异构体对组成的材料)形成结晶基序,模仿其外消旋对应物的反转关系。我们小组最近的研究探索了一个手性(N-苯甲酰基)甲基苄胺家族,以了解共结晶的结构边界。这项研究将这些较早的研究扩展到了包括萘甲酰胺基氨基甲酸酯类,其中分子骨架的体积比以前的二芳基酰胺类大约20%。制备了萘甲酰胺族,其中侧基官能团的大小逐渐增加(即,H至C 6 H 5)给出55种可能的外消旋和准外消旋组合的独特对。使用X射线晶体学,热分析方法和晶格能量计算从这些材料中收集的数据为重要的洞察力,在于空间更大的萘酰胺分子框架如何在准对映异构体的成对组装过程中促进取代基的更大结构变异。
更新日期:2020-11-25
中文翻译:

增加准外消旋物形成的结构边界:4-取代的萘甲酰胺
准外消旋物(由接近对映异构体对组成的材料)形成结晶基序,模仿其外消旋对应物的反转关系。我们小组最近的研究探索了一个手性(N-苯甲酰基)甲基苄胺家族,以了解共结晶的结构边界。这项研究将这些较早的研究扩展到了包括萘甲酰胺基氨基甲酸酯类,其中分子骨架的体积比以前的二芳基酰胺类大约20%。制备了萘甲酰胺族,其中侧基官能团的大小逐渐增加(即,H至C 6 H 5)给出55种可能的外消旋和准外消旋组合的独特对。使用X射线晶体学,热分析方法和晶格能量计算从这些材料中收集的数据为重要的洞察力,在于空间更大的萘酰胺分子框架如何在准对映异构体的成对组装过程中促进取代基的更大结构变异。



























 京公网安备 11010802027423号
京公网安备 11010802027423号