当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Absolute Trends and Accurate and Precise Gas-Phase Binding Energies of 1-Alkyl-3-Methylimidazolium Tetrafluoroborate Ionic Liquid Clusters from Combined Independent and Competitive TCID Measurements
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2020-11-24 , DOI: 10.1021/acs.jpca.0c07246 H. A. Roy 1 , M. T. Rodgers 1
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2020-11-24 , DOI: 10.1021/acs.jpca.0c07246 H. A. Roy 1 , M. T. Rodgers 1
Affiliation
|
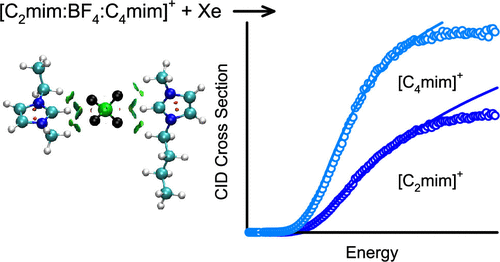
Ionic liquid (IL) development efforts have focused on achieving desired properties via tuning of the IL through variation of the cations and anions. However, works geared toward a microscopic understanding of the nature and strength of the intrinsic cation–anion interactions of ILs have been rather limited such that the intrinsic strength of the cation–anion interactions in ILs is largely unknown. In previous work, we employed threshold collision-induced dissociation approaches supported and enhanced by electronic structure calculations to characterize the nature of the cation–anion interactions in and determine the bond dissociation energies (BDEs) of a series of four 2:1 clusters of 1-alkyl-3-methylimidazolium cations and tetrafluoroborate anions, [2Cnmim:BF4]+. The cation was varied over the series: 1-ethyl-3-methylimidazolium, [C2mim]+, 1-butyl-3-methylimidazolium, [C4mim]+, 1-hexyl-3-methylimidazolium, [C6mim]+, and 1-octyl-3-methylimidazolium, [C8mim]+, to determine the structural and energetic effects of the size of the 1-alkyl substituent on the binding. The variation in the strength of binding determined for these [2Cnmim:BF4]+ clusters was found to be similar in magnitude to the average experimental uncertainty in these determinations. To definitively establish an absolute order of binding among these [2Cnmim:BF4]+ clusters, we extend this work here to include competitive binding studies of three mixed 2:1 clusters of 1-alkyl-3-methylimidazolium cations and tetrafluoroborate anions, [Cn–2mim:BF4:Cnmim]+ for n = 4, 6, and 8. Importantly, the results of the present work simultaneously provide the absolute BDEs of these mixed [Cn–2mim:BF4:Cnmim]+ clusters and the absolute relative order of the intrinsic binding interactions as a function of the cation with significantly improved precision. Further, by combining the thermochemical results of the previous and present studies, the BDEs of the [2Cnmim:BF4]+ clusters are more accurately and precisely determined.
中文翻译:

通过独立和竞争性TCID组合测量得到的1-烷基-3-甲基咪唑四氟硼酸根离子液体团簇的绝对趋势以及精确和精确的气相结合能
离子液体(IL)的开发工作集中在通过改变阳离子和阴离子来调节IL来实现所需的性能。但是,旨在从微观上了解IL的固有阳离子-阴离子相互作用的性质和强度的工作非常有限,因此,IL的阳离子-阴离子相互作用的固有强度在很大程度上是未知的。在先前的工作中,我们采用了由电子结构计算支持和增强的阈值碰撞诱导解离方法,以表征阳离子-阴离子相互作用的性质并确定一系列四个1:1的2:1簇的键解离能(BDE)。 -烷基-3-甲基咪唑鎓阳离子和四氟硼酸根阴离子,[2C n mim:BF 4 ] +。阳离子在以下系列中变化:1-乙基-3-甲基咪唑鎓,[C 2 mim] +,1-丁基-3-甲基咪唑鎓,[C 4 mim] +,1-己基-3-甲基咪唑鎓,[C 6 mim ] +和1-辛基-3-甲基咪唑鎓盐[C 8 mim] +,以确定1-烷基取代基大小对结合的结构和能量影响。这些[2C n mim:BF 4 ] +的结合强度变化在这些测定中,发现聚类的大小与平均实验不确定性相似。为了确定在这些[2C n mim:BF 4 ] +簇之间的结合的绝对顺序,我们在这里扩展了这项工作,包括对1-烷基-3-甲基咪唑鎓阳离子和四氟硼酸根阴离子的三个2:1混合簇的竞争性结合研究。 ,[C n –2 mim:BF 4:C n mim] +表示n = 4、6和8。重要的是,本工作的结果同时提供了这些混合的[C n –2 mim:BF]的绝对BDE。4:C n mim]+团簇和固有结合相互作用的绝对相对顺序作为阳离子的函数,具有显着提高的精度。此外,通过结合先前和当前研究的热化学结果,可以更准确,更准确地确定[2C n mim:BF 4 ] +簇的BDE 。
更新日期:2020-12-10
中文翻译:

通过独立和竞争性TCID组合测量得到的1-烷基-3-甲基咪唑四氟硼酸根离子液体团簇的绝对趋势以及精确和精确的气相结合能
离子液体(IL)的开发工作集中在通过改变阳离子和阴离子来调节IL来实现所需的性能。但是,旨在从微观上了解IL的固有阳离子-阴离子相互作用的性质和强度的工作非常有限,因此,IL的阳离子-阴离子相互作用的固有强度在很大程度上是未知的。在先前的工作中,我们采用了由电子结构计算支持和增强的阈值碰撞诱导解离方法,以表征阳离子-阴离子相互作用的性质并确定一系列四个1:1的2:1簇的键解离能(BDE)。 -烷基-3-甲基咪唑鎓阳离子和四氟硼酸根阴离子,[2C n mim:BF 4 ] +。阳离子在以下系列中变化:1-乙基-3-甲基咪唑鎓,[C 2 mim] +,1-丁基-3-甲基咪唑鎓,[C 4 mim] +,1-己基-3-甲基咪唑鎓,[C 6 mim ] +和1-辛基-3-甲基咪唑鎓盐[C 8 mim] +,以确定1-烷基取代基大小对结合的结构和能量影响。这些[2C n mim:BF 4 ] +的结合强度变化在这些测定中,发现聚类的大小与平均实验不确定性相似。为了确定在这些[2C n mim:BF 4 ] +簇之间的结合的绝对顺序,我们在这里扩展了这项工作,包括对1-烷基-3-甲基咪唑鎓阳离子和四氟硼酸根阴离子的三个2:1混合簇的竞争性结合研究。 ,[C n –2 mim:BF 4:C n mim] +表示n = 4、6和8。重要的是,本工作的结果同时提供了这些混合的[C n –2 mim:BF]的绝对BDE。4:C n mim]+团簇和固有结合相互作用的绝对相对顺序作为阳离子的函数,具有显着提高的精度。此外,通过结合先前和当前研究的热化学结果,可以更准确,更准确地确定[2C n mim:BF 4 ] +簇的BDE 。


























 京公网安备 11010802027423号
京公网安备 11010802027423号