当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Development of a Scalable Synthesis of trans-4-Fluorocyclohexylamine via Directed Hydrogenation
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2020-11-23 , DOI: 10.1021/acs.oprd.0c00444 Joyce C. Leung 1 , Thach T. Nguyen 1 , Mariusz Krawiec 2 , Donghong A. Gao 2 , Jonathan T. Reeves 1
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2020-11-23 , DOI: 10.1021/acs.oprd.0c00444 Joyce C. Leung 1 , Thach T. Nguyen 1 , Mariusz Krawiec 2 , Donghong A. Gao 2 , Jonathan T. Reeves 1
Affiliation
|
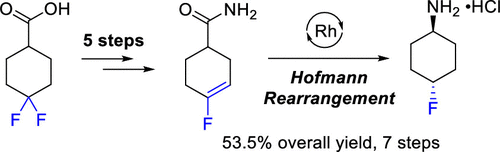
Herein, a scalable and practical process to prepare trans-4-fluorocyclohexylamine hydrochloride (1a) is described. By exploitation of the embedded gem-difluoride motif in the commercially available 4,4-difluorocyclohexanecarboxylic acid, a derived orthoester-masked acid underwent dehydrofluorination to provide the requisite vinyl fluoride for a directed hydrogenation event, enabling selective access to the trans-configuration of 1a.
中文翻译:

一个可扩展的综合开发跨通过定向加氢-4- Fluorocyclohexylamine
在此,描述了制备反式-4-氟环己胺盐酸盐(1a)的可扩展且实用的方法。通过利用市场上可买到的4,4-二氟环己烷甲酸中嵌入的宝石-二氟化物基序,对衍生自原酸酯掩蔽的酸进行脱氢氟化作用,以提供必要的氟乙烯用于定向氢化事件,从而能够选择性地获得1a的反式构型。
更新日期:2020-11-23
中文翻译:

一个可扩展的综合开发跨通过定向加氢-4- Fluorocyclohexylamine
在此,描述了制备反式-4-氟环己胺盐酸盐(1a)的可扩展且实用的方法。通过利用市场上可买到的4,4-二氟环己烷甲酸中嵌入的宝石-二氟化物基序,对衍生自原酸酯掩蔽的酸进行脱氢氟化作用,以提供必要的氟乙烯用于定向氢化事件,从而能够选择性地获得1a的反式构型。


























 京公网安备 11010802027423号
京公网安备 11010802027423号