当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of Reverse Glycosyl Fluorides and Rare Glycosyl Fluorides Enabled by Radical Decarboxylative Fluorination of Uronic Acids
Organic Letters ( IF 5.2 ) Pub Date : 2020-11-23 , DOI: 10.1021/acs.orglett.0c03514 Pengwei Chen 1, 2 , Peng Wang 1 , Qing Long 1 , Han Ding 1 , Guoqiang Cheng 1 , Tiantian Li 1 , Ming Li 1, 3, 4
Organic Letters ( IF 5.2 ) Pub Date : 2020-11-23 , DOI: 10.1021/acs.orglett.0c03514 Pengwei Chen 1, 2 , Peng Wang 1 , Qing Long 1 , Han Ding 1 , Guoqiang Cheng 1 , Tiantian Li 1 , Ming Li 1, 3, 4
Affiliation
|
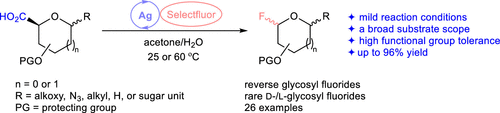
An efficient protocol for synthesizing reverse glycosyl fluorides is described, relying on silver-promoted decarboxylative fluorination of structurally diverse pentofuran- and hexopyranuronic acids under the mild reaction conditions. The potential applications of the reaction are further demonstrated by converting readily available d-uronic acid derivatives into uncommon d-/l-glycosyl fluorides through a C1-to-C5 switch strategy. The reaction mechanism is corroborated by 5-exo-trig radical cyclization of allyl α-d-C-glucopyranuronic acid triggered by decarboxylative fluorination.
中文翻译:

糖醛酸的自由基脱羧氟化合成逆糖基氟化物和稀有糖基氟化物
描述了一种有效的合成逆向糖基氟化物的方法,该方法依赖于在温和的反应条件下,银促进结构上多样的戊呋喃和六吡喃葡萄糖醛酸的脱羧氟化作用。该反应的潜在应用通过通过C1-C5转换策略将容易获得的d-糖醛酸衍生物转化为不常见的d - 1-糖苷氟来进一步证明。反应机理是通过5-证实外切-三角函数烯丙基α-的自由基环化d -C葡吡喃糖醛酸通过脱羧氟化触发。
更新日期:2020-12-04
中文翻译:

糖醛酸的自由基脱羧氟化合成逆糖基氟化物和稀有糖基氟化物
描述了一种有效的合成逆向糖基氟化物的方法,该方法依赖于在温和的反应条件下,银促进结构上多样的戊呋喃和六吡喃葡萄糖醛酸的脱羧氟化作用。该反应的潜在应用通过通过C1-C5转换策略将容易获得的d-糖醛酸衍生物转化为不常见的d - 1-糖苷氟来进一步证明。反应机理是通过5-证实外切-三角函数烯丙基α-的自由基环化d -C葡吡喃糖醛酸通过脱羧氟化触发。

























 京公网安备 11010802027423号
京公网安备 11010802027423号