当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Nickel-Catalyzed Regioselective Hydroarylation of Internal Enamides
Organic Letters ( IF 5.2 ) Pub Date : 2020-11-23 , DOI: 10.1021/acs.orglett.0c03542 Chenchen Wang 1 , Yang Xi 1 , Wenyi Huang 1 , Jingping Qu 1 , Yifeng Chen 1
Organic Letters ( IF 5.2 ) Pub Date : 2020-11-23 , DOI: 10.1021/acs.orglett.0c03542 Chenchen Wang 1 , Yang Xi 1 , Wenyi Huang 1 , Jingping Qu 1 , Yifeng Chen 1
Affiliation
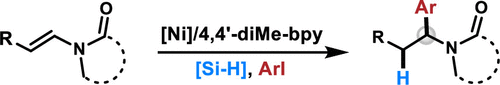
|
Herein, we disclose a nickel-catalyzed three-component reaction of internal enamide, diethoxymethylsilane, and aryl iodide to provide expedient access to benzylic amide derivatives. The protocol features a broad substrate scope with a moderate to excellent isolated yield under the mild condition. The high regioselectivity of Ni-catalyzed enamide hydroarylation can be attributed to the directing effect by the prefunctionalized nitrogen-containing group on the alkenes.
中文翻译:

镍催化内酰胺的区域选择性加氢芳基化
在本文中,我们公开了内部烯酰胺,二乙氧基甲基硅烷和芳基碘化物的镍催化的三组分反应,以提供对苄基酰胺衍生物的方便访问。该方案具有宽泛的底物范围,在温和条件下具有中等至优异的分离产率。Ni催化的酰胺酰胺氢芳基化的高区域选择性可以归因于烯烃上预官能化的含氮基团的导向作用。
更新日期:2020-12-04
中文翻译:

镍催化内酰胺的区域选择性加氢芳基化
在本文中,我们公开了内部烯酰胺,二乙氧基甲基硅烷和芳基碘化物的镍催化的三组分反应,以提供对苄基酰胺衍生物的方便访问。该方案具有宽泛的底物范围,在温和条件下具有中等至优异的分离产率。Ni催化的酰胺酰胺氢芳基化的高区域选择性可以归因于烯烃上预官能化的含氮基团的导向作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号