当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Novel 2-Aryloxazoline Compounds Exhibit an Inhibitory Effect on Candida spp., Including Antifungal-Resistant Isolates
ACS Medicinal Chemistry Letters ( IF 4.2 ) Pub Date : 2020-11-23 , DOI: 10.1021/acsmedchemlett.0c00449 Luis M Z Argomedo 1 , Vinicius M Barroso 2 , Cristiane S Barreiro 1 , Mariana P Darbem 1 , Kelly Ishida 2 , Hélio A Stefani 1
ACS Medicinal Chemistry Letters ( IF 4.2 ) Pub Date : 2020-11-23 , DOI: 10.1021/acsmedchemlett.0c00449 Luis M Z Argomedo 1 , Vinicius M Barroso 2 , Cristiane S Barreiro 1 , Mariana P Darbem 1 , Kelly Ishida 2 , Hélio A Stefani 1
Affiliation
|
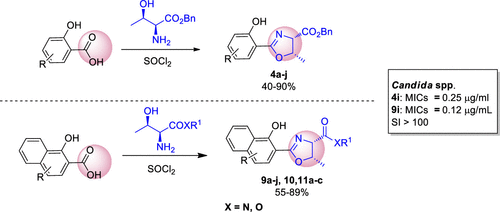
Because of the increased resistance to currently available antifungals, fungal infections represent a significant challenge to human health. Herein, we report the synthesis of 2-aryloxazoline derivatives from the reaction between l-threonine and derivatives of salicylic or naphthoic acid. In total, 26 compounds were obtained and tested against species of Candida, Cryptococcus, and Aspergillus. We found that all of the compounds inhibited the growth of Candida species at low concentrations (<0.25 μg/mL) and exhibited reduced hemolytic and cytotoxic activities. Additionally, compounds 4i and 9i were especially effective against antifungal-resistant isolates and the emerging fungus Candida auris. However, the compounds were less active on Cryptococcus and Aspergillus. Because of the improved in vitro antifungal efficacy and attenuated cytotoxicity, these two 2-aryloxazolines obtained from salicylic and naphthoic acid derivatives, respectively, may be considered lead molecules for the development of novel antifungal drugs.
中文翻译:

新型 2-芳基恶唑啉化合物对念珠菌具有抑制作用,包括抗真菌分离株
由于对目前可用的抗真菌药的抵抗力增加,真菌感染对人类健康构成了重大挑战。在此,我们报告了由l-苏氨酸与水杨酸或萘甲酸衍生物之间的反应合成 2-芳基恶唑啉衍生物。总共获得和反对的物种测试26种化合物念珠菌,隐球菌,和曲霉属。我们发现所有化合物在低浓度 (<0.25 μg/mL) 下都能抑制念珠菌的生长,并表现出降低的溶血和细胞毒性活性。此外,化合物4i和9i对抗真菌分离株和新兴真菌耳念珠菌特别有效。然而,这些化合物对隐球菌和曲霉的活性较低。由于体外抗真菌功效的提高和细胞毒性的减弱,这两种分别从水杨酸和萘甲酸衍生物中获得的 2-芳基恶唑啉可被认为是开发新型抗真菌药物的先导分子。
更新日期:2020-12-10
中文翻译:

新型 2-芳基恶唑啉化合物对念珠菌具有抑制作用,包括抗真菌分离株
由于对目前可用的抗真菌药的抵抗力增加,真菌感染对人类健康构成了重大挑战。在此,我们报告了由l-苏氨酸与水杨酸或萘甲酸衍生物之间的反应合成 2-芳基恶唑啉衍生物。总共获得和反对的物种测试26种化合物念珠菌,隐球菌,和曲霉属。我们发现所有化合物在低浓度 (<0.25 μg/mL) 下都能抑制念珠菌的生长,并表现出降低的溶血和细胞毒性活性。此外,化合物4i和9i对抗真菌分离株和新兴真菌耳念珠菌特别有效。然而,这些化合物对隐球菌和曲霉的活性较低。由于体外抗真菌功效的提高和细胞毒性的减弱,这两种分别从水杨酸和萘甲酸衍生物中获得的 2-芳基恶唑啉可被认为是开发新型抗真菌药物的先导分子。



























 京公网安备 11010802027423号
京公网安备 11010802027423号