当前位置:
X-MOL 学术
›
FEBS Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Wide‐open conformation of UDP‐MurNc‐tripeptide ligase revealed by the substrate‐free structure of MurE from Acinetobacter baumannii
FEBS Letters ( IF 3.5 ) Pub Date : 2020-12-03 , DOI: 10.1002/1873-3468.14007 Kyoung Ho Jung 1, 2 , Yeon-Gil Kim 3 , Chang Min Kim 1, 2 , Hyun Ji Ha 1, 2 , Chang Sup Lee 4 , Jun Hyuck Lee 5, 6 , Hyun Ho Park 1, 2
FEBS Letters ( IF 3.5 ) Pub Date : 2020-12-03 , DOI: 10.1002/1873-3468.14007 Kyoung Ho Jung 1, 2 , Yeon-Gil Kim 3 , Chang Min Kim 1, 2 , Hyun Ji Ha 1, 2 , Chang Sup Lee 4 , Jun Hyuck Lee 5, 6 , Hyun Ho Park 1, 2
Affiliation
|
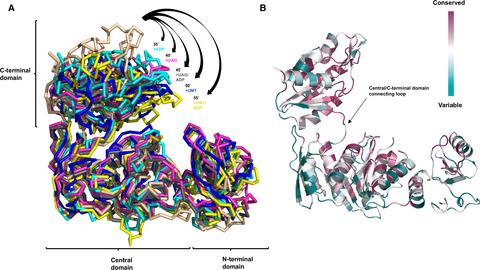
MurE ligase catalyzes the attachment of meso‐diaminopimelic acid to the UDP‐MurNAc‐l‐Ala‐d‐Glu using ATP and producing UDP‐MurNAc‐l‐Ala‐d‐Glu‐meso‐A2pm during bacterial cell wall biosynthesis. Owing to the critical role of this enzyme, MurE is considered an attractive target for antibacterial drugs. Despite extensive studies on MurE ligase, the structural dynamics of its conformational changes are still elusive. In this study, we present the substrate‐free structure of MurE from Acinetobacter baumannii, which is an antibiotic‐resistant superbacterium that has threatened global public health. The structure revealed that MurE has a wide‐open conformation and undergoes wide‐open, intermediately closed, and fully closed dynamic conformational transition. Unveiling structural dynamics of MurE will help to understand the working mechanism of this ligase and to design next‐generation antibiotics targeting MurE.
中文翻译:

鲍曼不动杆菌 MurE 无底物结构揭示的 UDP-MurNc-三肽连接酶的全开放构象
MurE 连接酶使用 ATP 催化内消旋二氨基庚二酸与 UDP-MurNAc-l-Ala-d-Glu 的连接,并在细菌细胞壁生物合成过程中产生 UDP-MurNAc-l-Ala-d-Glu-meso-A2pm。由于这种酶的关键作用,MurE 被认为是抗菌药物的有吸引力的靶点。尽管对 MurE 连接酶进行了广泛的研究,但其构象变化的结构动力学仍然难以捉摸。在这项研究中,我们展示了来自鲍曼不动杆菌的 MurE 的无底物结构,这是一种威胁全球公共健康的抗生素抗性超级细菌。该结构表明 MurE 具有全开构象,并经历全开、中闭和全闭动态构象转变。
更新日期:2020-12-03
中文翻译:

鲍曼不动杆菌 MurE 无底物结构揭示的 UDP-MurNc-三肽连接酶的全开放构象
MurE 连接酶使用 ATP 催化内消旋二氨基庚二酸与 UDP-MurNAc-l-Ala-d-Glu 的连接,并在细菌细胞壁生物合成过程中产生 UDP-MurNAc-l-Ala-d-Glu-meso-A2pm。由于这种酶的关键作用,MurE 被认为是抗菌药物的有吸引力的靶点。尽管对 MurE 连接酶进行了广泛的研究,但其构象变化的结构动力学仍然难以捉摸。在这项研究中,我们展示了来自鲍曼不动杆菌的 MurE 的无底物结构,这是一种威胁全球公共健康的抗生素抗性超级细菌。该结构表明 MurE 具有全开构象,并经历全开、中闭和全闭动态构象转变。


























 京公网安备 11010802027423号
京公网安备 11010802027423号