当前位置:
X-MOL 学术
›
Propellants Explos. Pyrotech.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Heat of Formation of Triazole‐Based Salts: Prediction and Experimental Validation
Propellants, Explosives, Pyrotechnics ( IF 1.8 ) Pub Date : 2020-11-24 , DOI: 10.1002/prep.202000187 Julien Glorian 1 , Kyung‐Tae Han 1 , Silke Braun 1 , Barbara Baschung 1
Propellants, Explosives, Pyrotechnics ( IF 1.8 ) Pub Date : 2020-11-24 , DOI: 10.1002/prep.202000187 Julien Glorian 1 , Kyung‐Tae Han 1 , Silke Braun 1 , Barbara Baschung 1
Affiliation
|
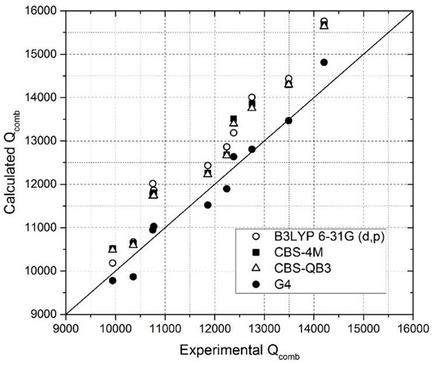
This work contributes to the growing interest in predictions linked with energetic salts. A reliable method to accurately compute the heat of formation of triazole‐based salts was investigated. Calculations were based on Born‐Haber energy cycles: gas‐phase enthalpy of ions and lattice enthalpy were calculated separately. Ten triazole‐based salts were synthesized and fully characterized. Their heat of combustion was measured by bomb calorimeter. Gas‐phase heat of formation of cations and anions were computed at four different levels of theory: B3LYP 6‐31G(d,p), CBS‐4M, CBS‐QB3, and G4. Ionic volumes were calculated at the B3LYP 6‐31G(d,p) level with and without corrections. Lattice enthalpy estimations, based on calculated ionic volumes, were performed with the help of Jenkins and Gutowski methods. Combinations of the obtained results (gas‐phase enthalpy of ions and lattice enthalpy) were used in the Born‐Haber approach to predict solid phase enthalpy of formation of studied energetic salts. Direct comparison with experimental measurements enabled the identification of the most reliable path for energetic salt standard enthalpy of formation prediction.
中文翻译:

三唑基盐形成的热量:预测和实验验证
这项工作促使人们对与高能盐有关的预测越来越感兴趣。研究了一种准确计算三唑基盐形成热的可靠方法。计算基于Born-Haber能量循环:分别计算离子的气相焓和晶格焓。合成了十种基于三唑的盐并进行了充分表征。用炸弹量热计测量它们的燃烧热。在四个不同的理论水平上计算了阳离子和阴离子形成的气相热:B3LYP 6-31G(d,p),CBS-4M,CBS-QB3和G4。离子量是在B3LYP 6-31G(d,p)水平下计算的,有和没有进行校正。借助詹金斯(Jenkins)和古托夫斯基(Gutowski)方法,基于计算出的离子体积进行了晶格焓的估算。Born-Haber方法将获得的结果(离子的气相焓和晶格焓)的组合用于预测所研究的高能盐形成的固相焓。与实验测量值的直接比较能够确定高能盐标准生成焓预测的最可靠途径。
更新日期:2021-01-11
中文翻译:

三唑基盐形成的热量:预测和实验验证
这项工作促使人们对与高能盐有关的预测越来越感兴趣。研究了一种准确计算三唑基盐形成热的可靠方法。计算基于Born-Haber能量循环:分别计算离子的气相焓和晶格焓。合成了十种基于三唑的盐并进行了充分表征。用炸弹量热计测量它们的燃烧热。在四个不同的理论水平上计算了阳离子和阴离子形成的气相热:B3LYP 6-31G(d,p),CBS-4M,CBS-QB3和G4。离子量是在B3LYP 6-31G(d,p)水平下计算的,有和没有进行校正。借助詹金斯(Jenkins)和古托夫斯基(Gutowski)方法,基于计算出的离子体积进行了晶格焓的估算。Born-Haber方法将获得的结果(离子的气相焓和晶格焓)的组合用于预测所研究的高能盐形成的固相焓。与实验测量值的直接比较能够确定高能盐标准生成焓预测的最可靠途径。



























 京公网安备 11010802027423号
京公网安备 11010802027423号