当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
2‐ and 2,7‐Substituted para‐N‐Methylpyridinium Pyrenes: Syntheses, Molecular and Electronic Structures, Photophysical, Electrochemical, and Spectroelectrochemical Properties and Binding to Double‐Stranded (ds) DNA
Chemistry - A European Journal ( IF 4.3 ) Pub Date : 2020-11-24 , DOI: 10.1002/chem.202004748 Goutam Kumar Kole 1, 2 , Julia Merz 1 , Anissa Amar 3 , Bruno Fontaine 4 , Abdou Boucekkine 4 , Jörn Nitsch 1 , Sabine Lorenzen 1 , Alexandra Friedrich 1 , Ivo Krummenacher 1 , Marta Košćak 5 , Holger Braunschweig 1 , Ivo Piantanida 5 , Jean-François Halet 4 , Klaus Müller-Buschbaum 6 , Todd B Marder 1
Chemistry - A European Journal ( IF 4.3 ) Pub Date : 2020-11-24 , DOI: 10.1002/chem.202004748 Goutam Kumar Kole 1, 2 , Julia Merz 1 , Anissa Amar 3 , Bruno Fontaine 4 , Abdou Boucekkine 4 , Jörn Nitsch 1 , Sabine Lorenzen 1 , Alexandra Friedrich 1 , Ivo Krummenacher 1 , Marta Košćak 5 , Holger Braunschweig 1 , Ivo Piantanida 5 , Jean-François Halet 4 , Klaus Müller-Buschbaum 6 , Todd B Marder 1
Affiliation
|
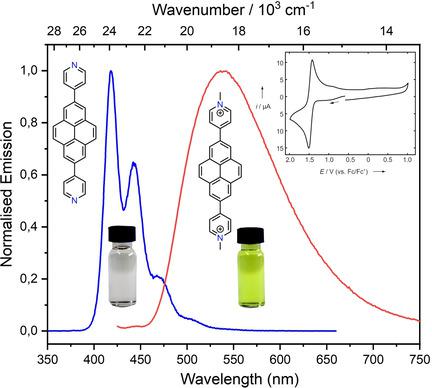
Two N‐methylpyridinium compounds and analogous N‐protonated salts of 2‐ and 2,7‐substituted 4‐pyridyl‐pyrene compounds were synthesised and their crystal structures, photophysical properties both in solution and in the solid state, electrochemical and spectroelectrochemical properties were studied. Upon methylation or protonation, the emission maxima are significantly bathochromically shifted compared to the neutral compounds, although the absorption maxima remain almost unchanged. As a result, the cationic compounds show very large apparent Stokes shifts of up to 7200 cm−1. The N‐methylpyridinium compounds have a single reduction at ca. −1.5 V vs. Fc/Fc+ in MeCN. While the reduction process was reversible for the 2,7‐disubstituted compound, it was irreversible for the mono‐substituted one. Experimental findings are complemented by DFT and TD‐DFT calculations. Furthermore, the N‐methylpyridinium compounds show strong interactions with calf thymus (ct)‐DNA, presumably by intercalation, which paves the way for further applications of these multi‐functional compounds as potential DNA‐bioactive agents.
中文翻译:

2- 和 2,7- 取代的对-N-甲基吡啶芘:合成、分子和电子结构、光物理、电化学和光谱电化学特性以及与双链 (ds) DNA 的结合
合成了两种N-甲基吡啶鎓化合物以及类似的2-和2,7-取代4-吡啶基芘化合物的N-质子化盐,并研究了它们的晶体结构、溶液和固态的光物理性质、电化学和光谱电化学性质。甲基化或质子化时,与中性化合物相比,发射最大值显着红移,尽管吸收最大值几乎保持不变。结果,阳离子化合物显示出高达7200cm -1的非常大的表观斯托克斯位移。N-甲基吡啶鎓化合物在约处有一次还原。-1.5 V vs. MeCN 中的 Fc/Fc +。虽然还原过程对于 2,7-二取代化合物来说是可逆的,但对于单取代化合物来说是不可逆的。实验结果得到了 DFT 和 TD-DFT 计算的补充。此外,N-甲基吡啶鎓化合物与小牛胸腺 (ct)-DNA 表现出强烈的相互作用,可能是通过嵌入,这为这些多功能化合物作为潜在 DNA 生物活性剂的进一步应用铺平了道路。
更新日期:2020-11-24
中文翻译:

2- 和 2,7- 取代的对-N-甲基吡啶芘:合成、分子和电子结构、光物理、电化学和光谱电化学特性以及与双链 (ds) DNA 的结合
合成了两种N-甲基吡啶鎓化合物以及类似的2-和2,7-取代4-吡啶基芘化合物的N-质子化盐,并研究了它们的晶体结构、溶液和固态的光物理性质、电化学和光谱电化学性质。甲基化或质子化时,与中性化合物相比,发射最大值显着红移,尽管吸收最大值几乎保持不变。结果,阳离子化合物显示出高达7200cm -1的非常大的表观斯托克斯位移。N-甲基吡啶鎓化合物在约处有一次还原。-1.5 V vs. MeCN 中的 Fc/Fc +。虽然还原过程对于 2,7-二取代化合物来说是可逆的,但对于单取代化合物来说是不可逆的。实验结果得到了 DFT 和 TD-DFT 计算的补充。此外,N-甲基吡啶鎓化合物与小牛胸腺 (ct)-DNA 表现出强烈的相互作用,可能是通过嵌入,这为这些多功能化合物作为潜在 DNA 生物活性剂的进一步应用铺平了道路。


























 京公网安备 11010802027423号
京公网安备 11010802027423号