International Journal of Hydrogen Energy ( IF 7.2 ) Pub Date : 2020-11-24 , DOI: 10.1016/j.ijhydene.2020.11.008 Shasha Yang , Dewei Rao , Jingjing Ye , Shaokang Yang , Chaonan Zhang , Can Gao , Xuecheng Zhou , Huan Yang , Xiaohong Yan
|
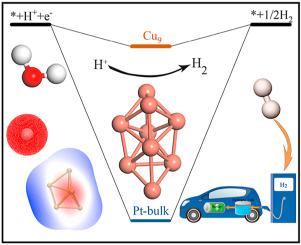
Studying the hydrogen evolution reaction (HER) catalyst is important for the global energy crisis. Clusters have many special characteristics due to quantum size effect and super high specific surface area, including optical performance, catalytic performance, etc. In this work, the structures of transition metal cluster TMn (TM = Co, Ni, Cu, Pd, Pt, n = 4–10) were searched and optimized by quantum chemistry methods. To search for non-precious metal catalysts, we calculated the Gibbs free energies for HER process on different clusters. Furthermore, the electronic structures of clusters before and after the reaction with H were analyzed, including the molecular surface electron distribution, the frontier molecular orbital, and the charge transfer properties, which dominated the HER processes. The results show that the Cu clusters have excellent HER catalytic properties due to its suitable surface electron distribution and HOMO/LUMO levels, especially Cu4, Cu7 and Cu9, which even comparable to Pt catalysts. These results can help us better understand the mechanism of clusters catalyze HER process.
中文翻译:

过渡金属簇催化剂催化析氢的机理
研究氢气析出反应(HER)催化剂对全球能源危机至关重要。由于量子尺寸效应和超高比表面积,团簇具有许多特殊的特性,包括光学性能,催化性能等。在这项工作中,过渡金属团簇TM n的结构(TM = Co,Ni,Cu,Pd,Pt,n = 4-10)通过量子化学方法进行了搜索和优化。为了搜索非贵金属催化剂,我们计算了在不同簇上进行HER过程的吉布斯自由能。此外,还分析了与H反应前后的簇的电子结构,包括分子表面电子分布,前沿分子轨道和电荷转移性质,这些都主导了HER过程。结果表明,Cu簇由于具有合适的表面电子分布和HOMO / LUMO能级而具有优异的HER催化性能,尤其是Cu 4,Cu 7和Cu 9。,甚至可以与铂催化剂媲美。这些结果可以帮助我们更好地了解簇催化HER过程的机理。


























 京公网安备 11010802027423号
京公网安备 11010802027423号