当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Stereocontrolled Total Synthesis of (−)-Isocelorbicol and Its Elaboration to Natural Dihydro-β-agarofuran Esters
Organic Letters ( IF 5.2 ) Pub Date : 2020-11-23 , DOI: 10.1021/acs.orglett.0c03419 Tomoyo Mohri 1 , Yusuke Takahashi 1 , Eunsang Kwon 2 , Shigefumi Kuwahara 1 , Yusuke Ogura 1, 3
Organic Letters ( IF 5.2 ) Pub Date : 2020-11-23 , DOI: 10.1021/acs.orglett.0c03419 Tomoyo Mohri 1 , Yusuke Takahashi 1 , Eunsang Kwon 2 , Shigefumi Kuwahara 1 , Yusuke Ogura 1, 3
Affiliation
|
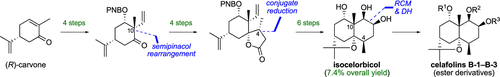
The first total synthesis of four naturally occurring dihydro-β-agarofuran esters has been accomplished via a highly stereocontrolled 14-step access to their common core triol, (−)-isocelorbicol. A semipinacol rearrangement of an epoxy alcohol to install a quaternary carbon, diastereoselective conjugate reduction of a spirocyclic butenolide for the establishment of a methyl-bearing chiral center, and ring-closing metathesis to construct the decalin ring system were exploited as the key steps for the high-yielding synthesis of (−)-isocelorbicol.
中文翻译:

立体控制的(-)-异氟比索的全合成及其对天然二氢-β-agarofuran酯的修饰
四种天然存在的二氢-β-琼脂呋喃酯的第一个全合成反应是通过高度立体控制的14步进入其常见的核心三醇(-)-异celicbicol。环氧醇的半频醇重排以安装季碳,螺环丁烯内酯的非对映选择性共轭还原以建立带有甲基的手性中心,以及闭环易位来构造十氢化萘环体系。 (-)-异青霉酚的高产率合成。
更新日期:2020-12-04
中文翻译:

立体控制的(-)-异氟比索的全合成及其对天然二氢-β-agarofuran酯的修饰
四种天然存在的二氢-β-琼脂呋喃酯的第一个全合成反应是通过高度立体控制的14步进入其常见的核心三醇(-)-异celicbicol。环氧醇的半频醇重排以安装季碳,螺环丁烯内酯的非对映选择性共轭还原以建立带有甲基的手性中心,以及闭环易位来构造十氢化萘环体系。 (-)-异青霉酚的高产率合成。



























 京公网安备 11010802027423号
京公网安备 11010802027423号