当前位置:
X-MOL 学术
›
Chem. Res. Toxicol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Analysis of Nicotine and Non-nicotine Tobacco Constituents in Aqueous Smoke/Aerosol Extracts by UHPLC and Ultraperformance Convergence Chromatography–Tandem Mass Spectrometry
Chemical Research in Toxicology ( IF 4.1 ) Pub Date : 2020-11-23 , DOI: 10.1021/acs.chemrestox.0c00312 Melanie A Rehder Silinski 1 , Teruyo Uenoyama 1 , Donna P Coleman 1 , James C Blake 1 , Brian F Thomas 1 , Julie A Marusich 1 , Kia J Jackson 2 , Steven E Meredith 2 , Robert F Gahl 2
Chemical Research in Toxicology ( IF 4.1 ) Pub Date : 2020-11-23 , DOI: 10.1021/acs.chemrestox.0c00312 Melanie A Rehder Silinski 1 , Teruyo Uenoyama 1 , Donna P Coleman 1 , James C Blake 1 , Brian F Thomas 1 , Julie A Marusich 1 , Kia J Jackson 2 , Steven E Meredith 2 , Robert F Gahl 2
Affiliation
|
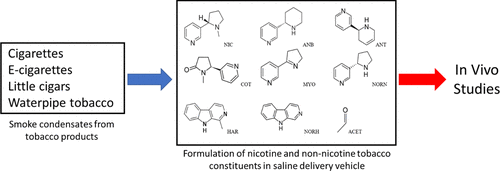
The non-nicotine constituents of tobacco may alter the reinforcing effects of nicotine, but the quantitative and qualitative profiles of these chemicals in tobacco products such as electronic cigarettes (e-cigarettes), cigars, and waterpipe tobacco are not well characterized. The objective of this work was to develop and validate analytical methods to utilize saline both as an extraction solvent for smoke condensates from cigarettes, little cigars, and waterpipe tobacco and aerosols from e-cigarettes and as a delivery vehicle of nicotine and non-nicotine constitents for nonclinical pharmacological studies. Ultrahigh-performance liquid chromatography was used to analyze nicotine and acetaldehyde, and a novel ultraperformance convergence chromatography–tandem mass spectrometry method was developed to analyze anabasine, anatabine, cotinine, myosmine, nornicotine, harmane, and norharmane. Linearity was confirmed for each standard curve with correlation coefficients (r) ≥ 0.99, and relative errors (RE) for the standards were ≤±10% over the calibration ranges. Method validation was performed by preparing triplicate samples in saline to mimic the composition and concentration of each analyte in the smoke or aerosol condensate and were used to determine method accuracy and precision. Relative standard deviation values were ≤15% and mean RE ≤15% for each analyte at each concentration level. Selectivity of the methods was demonstrated by the absence of peaks in blank vehicle or diluent samples. Storage stability was assessed over ∼45 days. Precision (%RSD ≤ 13) and recovery (percent of day 0 ≥ 80%) indicated that the saline formulations of all four products could be considered stable for up to ∼45 days at 4–8 °C. Therefore, the use of saline both as an extraction solvent and as a delivery vehicle adds versatility and improved performance in the study of the pharmacological effects of constituents from mainstream smoke and aerosols generated from cigarettes, little cigars, waterpipes, and e-cigarettes.
中文翻译:

使用 UHPLC 和超高效合相色谱-串联质谱法分析水性烟雾/气溶胶提取物中的尼古丁和非尼古丁烟草成分
烟草的非尼古丁成分可能会改变尼古丁的增强作用,但这些化学物质在电子烟(电子烟)、雪茄和水烟烟草等烟草产品中的定量和定性特征尚未得到很好的表征。这项工作的目的是开发和验证分析方法,以利用盐水作为香烟、小雪茄和水烟烟草中的烟雾冷凝物和电子烟气雾剂的提取溶剂,以及作为尼古丁和非尼古丁成分的传递载体用于非临床药理学研究。使用超高效液相色谱分析尼古丁和乙醛,开发了一种新型的超高效合相色谱-串联质谱法来分析大麻素、大麻素、可替宁、肌碱、去甲烟碱、甘烷和去甲甘烷。用相关系数(r) ≥ 0.99,并且标准的相对误差 (RE) 在校准范围内≤±10%。方法验证通过在盐水中制备一式三份样品来模拟烟雾或气溶胶冷凝物中每种分析物的组成和浓度来进行,并用于确定方法的准确性和精密度。对于每个浓度水平的每种分析物,相对标准偏差值≤15%,平均RE≤15%。这些方法的选择性通过空白载体或稀释剂样品中没有峰来证明。在大约 45 天内评估了储存稳定性。精密度 (%RSD ≤ 13) 和回收率(第 0 天的百分比≥ 80%)表明,所有四种产品的盐水配方在 4–8 °C 下可稳定长达 45 天。所以,
更新日期:2020-12-21
中文翻译:

使用 UHPLC 和超高效合相色谱-串联质谱法分析水性烟雾/气溶胶提取物中的尼古丁和非尼古丁烟草成分
烟草的非尼古丁成分可能会改变尼古丁的增强作用,但这些化学物质在电子烟(电子烟)、雪茄和水烟烟草等烟草产品中的定量和定性特征尚未得到很好的表征。这项工作的目的是开发和验证分析方法,以利用盐水作为香烟、小雪茄和水烟烟草中的烟雾冷凝物和电子烟气雾剂的提取溶剂,以及作为尼古丁和非尼古丁成分的传递载体用于非临床药理学研究。使用超高效液相色谱分析尼古丁和乙醛,开发了一种新型的超高效合相色谱-串联质谱法来分析大麻素、大麻素、可替宁、肌碱、去甲烟碱、甘烷和去甲甘烷。用相关系数(r) ≥ 0.99,并且标准的相对误差 (RE) 在校准范围内≤±10%。方法验证通过在盐水中制备一式三份样品来模拟烟雾或气溶胶冷凝物中每种分析物的组成和浓度来进行,并用于确定方法的准确性和精密度。对于每个浓度水平的每种分析物,相对标准偏差值≤15%,平均RE≤15%。这些方法的选择性通过空白载体或稀释剂样品中没有峰来证明。在大约 45 天内评估了储存稳定性。精密度 (%RSD ≤ 13) 和回收率(第 0 天的百分比≥ 80%)表明,所有四种产品的盐水配方在 4–8 °C 下可稳定长达 45 天。所以,



























 京公网安备 11010802027423号
京公网安备 11010802027423号