当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enhanced Reactivity and Primary Liquid-Phase Forming in Mechanochemically Activated Soda–Lime–Silica Glass Batches
ACS Sustainable Chemistry & Engineering ( IF 8.4 ) Pub Date : 2020-11-23 , DOI: 10.1021/acssuschemeng.0c05980 Perla P. Rodríguez-Salazar 1 , Gregorio Vargas 1 , Saúl R. Ruíz-Ontiveros 2 , Oswaldo Burciaga-Díaz 3 , Sagrario M. Montemayor 4 , Antonio F. Fuentes 1
ACS Sustainable Chemistry & Engineering ( IF 8.4 ) Pub Date : 2020-11-23 , DOI: 10.1021/acssuschemeng.0c05980 Perla P. Rodríguez-Salazar 1 , Gregorio Vargas 1 , Saúl R. Ruíz-Ontiveros 2 , Oswaldo Burciaga-Díaz 3 , Sagrario M. Montemayor 4 , Antonio F. Fuentes 1
Affiliation
|
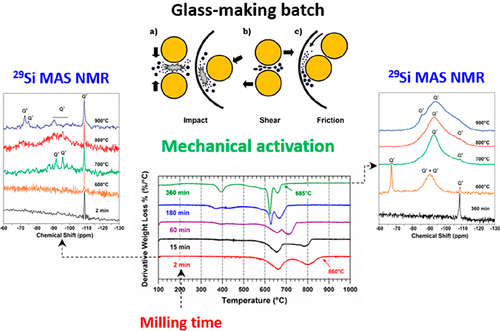
Glass manufacturing is a high-temperature, energy-intensive, and highly inefficient process, with a significant global environmental impact. Therefore, given the high-scaled nature of the glass-making sector, the design of more energy-efficient manufacturing protocols is an economic and environmental imperative. In this context, this work presents a comprehensive study about the milling effect in the heat-driven chemistry and phase evolution, of a typical soda–lime–silica (Na2O:CaO:SiO2) flat glass batch. Our findings show that, with the experimental settings imposed in this work, mechanochemical reactions leading to in situ formation of alkali/alkaline-earth silicate compounds in the vial, are most likely absent. However, all carbonated raw materials of the formula (soda ash, calcite, and dolomite), undergo severe structural damage during milling and even become amorphous to XRD after milling for 360 min, with their decomposition reactions shifting markedly toward lower temperatures as the milling time increases (e.g., the volatile fraction of the formula activated for 360 min, is completely removed almost 200 °C below than of a nonactivated reference sample). Furthermore, milling has also a profound effect in the evolution of the silicon local coordination environment, with temperature, and in the formation temperature and stoichiometry of silicate species observed on heating. Moreover, events related to the depolymerization of the fully linked Q4 units characteristic of crystalline SiO2 (i.e., formation of metal silicates), are observed in activated batches at temperatures well below those of the reference sample (e.g., 600 °C when activated for 360 min vs 900 °C of a nonactivated sample). These changes are not only a consequence of particle size reduction, because the particle size distribution of the batch does not change much after 30 min; milling-induced amorphization of the carbonated fraction of the formula and the corresponding reduced activation energy for their decomposition reactions and improved reactivity toward SiO2 are the driving forces behind the significant reduction in the formation temperature of the first liquid phases with increasing milling time.
中文翻译:

机械化学活化的苏打水,石灰玻璃,硅玻璃批次中增强的反应性和初级液相形成
玻璃制造是高温,高能耗,低效率的过程,对全球环境产生重大影响。因此,考虑到玻璃制造行业的规模化性质,设计更加节能的制造协议对经济和环境至关重要。在这种情况下,这项工作提出了关于典型的钠钙石灰硅(Na 2 O:CaO:SiO 2)平板玻璃批次。我们的发现表明,在这项工作中强加了实验设置,很可能不存在导致小瓶中原位形成碱/碱土金属硅酸盐化合物的机械化学反应。但是,所有具有化学式的碳酸盐原料(苏打灰,方解石和白云石)在研磨过程中会遭受严重的结构破坏,并且在研磨360分钟后甚至对XRD变成非晶态,随着研磨时间的延长,它们的分解反应会明显向低温转移增加(例如,在360分钟内激活的配方中的挥发性部分,比未激活的参考样品低了近200°C,就被完全去除了)。此外,随着温度,温度,加热时观察到的硅酸盐种类的形成温度和化学计量。此外,与完全连接的Q的解聚有关的事件在远远低于参考样品的温度下,在活化批次中观察到4个单位的结晶性SiO 2特性(即金属硅酸盐的形成)(例如,活化360分钟时为600°C,而未活化样品为900°C) 。这些变化不仅是粒径减小的结果,因为批料的粒径分布在30分钟后变化不大。研磨导致的式的碳酸部分的非晶化以及其分解反应相应降低的活化能以及提高的对SiO 2的反应性是随着研磨时间的增加显着降低第一液相的形成温度的驱动力。
更新日期:2020-12-07
中文翻译:

机械化学活化的苏打水,石灰玻璃,硅玻璃批次中增强的反应性和初级液相形成
玻璃制造是高温,高能耗,低效率的过程,对全球环境产生重大影响。因此,考虑到玻璃制造行业的规模化性质,设计更加节能的制造协议对经济和环境至关重要。在这种情况下,这项工作提出了关于典型的钠钙石灰硅(Na 2 O:CaO:SiO 2)平板玻璃批次。我们的发现表明,在这项工作中强加了实验设置,很可能不存在导致小瓶中原位形成碱/碱土金属硅酸盐化合物的机械化学反应。但是,所有具有化学式的碳酸盐原料(苏打灰,方解石和白云石)在研磨过程中会遭受严重的结构破坏,并且在研磨360分钟后甚至对XRD变成非晶态,随着研磨时间的延长,它们的分解反应会明显向低温转移增加(例如,在360分钟内激活的配方中的挥发性部分,比未激活的参考样品低了近200°C,就被完全去除了)。此外,随着温度,温度,加热时观察到的硅酸盐种类的形成温度和化学计量。此外,与完全连接的Q的解聚有关的事件在远远低于参考样品的温度下,在活化批次中观察到4个单位的结晶性SiO 2特性(即金属硅酸盐的形成)(例如,活化360分钟时为600°C,而未活化样品为900°C) 。这些变化不仅是粒径减小的结果,因为批料的粒径分布在30分钟后变化不大。研磨导致的式的碳酸部分的非晶化以及其分解反应相应降低的活化能以及提高的对SiO 2的反应性是随着研磨时间的增加显着降低第一液相的形成温度的驱动力。


























 京公网安备 11010802027423号
京公网安备 11010802027423号