当前位置:
X-MOL 学术
›
Adv. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Fullerene Desymmetrization as a Means to Achieve Single‐Enantiomer Electron Acceptors with Maximized Chiroptical Responsiveness
Advanced Materials ( IF 29.4 ) Pub Date : 2020-11-23 , DOI: 10.1002/adma.202004115 Wenda Shi 1 , Francesco Salerno 1, 2 , Matthew D. Ward 2, 3 , Alejandro Santana‐Bonilla 1 , Jessica Wade 1, 2, 3 , Xueyan Hou 4 , Tong Liu 4 , T. John S. Dennis 4 , Alasdair J. Campbell 2, 3 , Kim E. Jelfs 1, 2 , Matthew J. Fuchter 1
Advanced Materials ( IF 29.4 ) Pub Date : 2020-11-23 , DOI: 10.1002/adma.202004115 Wenda Shi 1 , Francesco Salerno 1, 2 , Matthew D. Ward 2, 3 , Alejandro Santana‐Bonilla 1 , Jessica Wade 1, 2, 3 , Xueyan Hou 4 , Tong Liu 4 , T. John S. Dennis 4 , Alasdair J. Campbell 2, 3 , Kim E. Jelfs 1, 2 , Matthew J. Fuchter 1
Affiliation
|
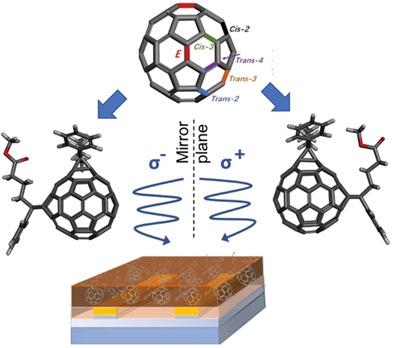
Solubilized fullerene derivatives have revolutionized the development of organic photovoltaic devices, acting as excellent electron acceptors. The addition of solubilizing addends to the fullerene cage results in a large number of isomers, which are generally employed as isomeric mixtures. Moreover, a significant number of these isomers are chiral, which further adds to the isomeric complexity. The opportunities presented by single‐isomer, and particularly single‐enantiomer, fullerenes in organic electronic materials and devices are poorly understood however. Here, ten pairs of enantiomers are separated from the 19 structural isomers of bis[60]phenyl‐C61‐butyric acid methyl ester, using them to elucidate important chiroptical relationships and demonstrating their application to a circularly polarized light (CPL)‐detecting device. Larger chiroptical responses are found, occurring through the inherent chirality of the fullerene. When used in a single‐enantiomer organic field‐effect transistor, the potential to discriminate CPL with a fast light response time and with a very high photocurrent dissymmetry factor (gph = 1.27 ± 0.06) is demonstrated. This study thus provides key strategies to design fullerenes with large chiroptical responses for use as chiral components of organic electronic devices. It is anticipated that this data will position chiral fullerenes as an exciting material class for the growing field of chiral electronic technologies.
中文翻译:

富勒烯去对称化是获得具有最大手性响应性的单对映体电子受体的手段
可溶性富勒烯衍生物彻底改变了有机光伏器件的发展,成为了出色的电子受体。向富勒烯笼中添加可溶的加成物导致大量异构体,其通常用作异构体混合物。此外,这些异构体中有大量是手性的,这进一步增加了异构体的复杂性。但是,人们对有机电子材料和器件中的单一异构体(尤其是单一对映异构体)富勒烯所带来的机会知之甚少。在这里,从双[60]苯基-C61-丁酸甲酯的19个结构异构体中分离出十对对映体,用它们阐明重要的手性关系,并证明了它们在圆偏振光(CPL)检测装置中的应用。发现较大的手性反应,这是由于富勒烯的固有手性引起的。当用于单对映体有机场效应晶体管中时,以快速的光响应时间和非常高的光电流不对称因子来区分CPL的潜力(g ph = 1.27±0.06)。因此,这项研究为设计具有较大手性响应的富勒烯提供了关键策略,以用作有机电子器件的手性组分。预计该数据将使手性富勒烯成为手性电子技术不断发展的领域中令人兴奋的材料类别。
更新日期:2021-01-04
中文翻译:

富勒烯去对称化是获得具有最大手性响应性的单对映体电子受体的手段
可溶性富勒烯衍生物彻底改变了有机光伏器件的发展,成为了出色的电子受体。向富勒烯笼中添加可溶的加成物导致大量异构体,其通常用作异构体混合物。此外,这些异构体中有大量是手性的,这进一步增加了异构体的复杂性。但是,人们对有机电子材料和器件中的单一异构体(尤其是单一对映异构体)富勒烯所带来的机会知之甚少。在这里,从双[60]苯基-C61-丁酸甲酯的19个结构异构体中分离出十对对映体,用它们阐明重要的手性关系,并证明了它们在圆偏振光(CPL)检测装置中的应用。发现较大的手性反应,这是由于富勒烯的固有手性引起的。当用于单对映体有机场效应晶体管中时,以快速的光响应时间和非常高的光电流不对称因子来区分CPL的潜力(g ph = 1.27±0.06)。因此,这项研究为设计具有较大手性响应的富勒烯提供了关键策略,以用作有机电子器件的手性组分。预计该数据将使手性富勒烯成为手性电子技术不断发展的领域中令人兴奋的材料类别。



























 京公网安备 11010802027423号
京公网安备 11010802027423号