Molecular Cell ( IF 16.0 ) Pub Date : 2020-11-23 , DOI: 10.1016/j.molcel.2020.10.039 Goragot Wisedchaisri 1 , Lige Tonggu 1 , Tamer M Gamal El-Din 1 , Eedann McCord 1 , Ning Zheng 2 , William A Catterall 1
|
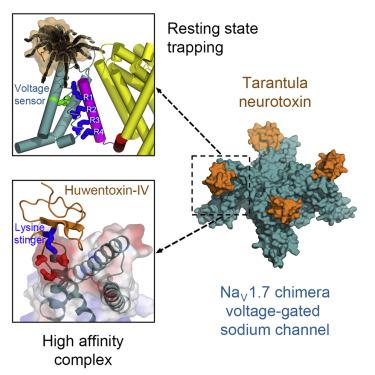
Voltage-gated sodium channels initiate electrical signals and are frequently targeted by deadly gating-modifier neurotoxins, including tarantula toxins, which trap the voltage sensor in its resting state. The structural basis for tarantula-toxin action remains elusive because of the difficulty of capturing the functionally relevant form of the toxin-channel complex. Here, we engineered the model sodium channel NaVAb with voltage-shifting mutations and the toxin-binding site of human NaV1.7, an attractive pain target. This mutant chimera enabled us to determine the cryoelectron microscopy (cryo-EM) structure of the channel functionally arrested by tarantula toxin. Our structure reveals a high-affinity resting-state-specific toxin-channel interaction between a key lysine residue that serves as a “stinger” and penetrates a triad of carboxyl groups in the S3-S4 linker of the voltage sensor. By unveiling this high-affinity binding mode, our studies establish a high-resolution channel-docking and resting-state locking mechanism for huwentoxin-IV and provide guidance for developing future resting-state-targeted analgesic drugs.
中文翻译:

狼蛛毒素高亲和力捕获静息状态下 NaV1.7 通道的结构基础
电压门控钠通道会启动电信号,并经常成为致命的门控修饰神经毒素的目标,包括狼蛛毒素,它会将电压传感器困在静止状态。由于难以捕获毒素通道复合物的功能相关形式,狼蛛毒素作用的结构基础仍然难以捉摸。在这里,我们设计了具有电压转换突变的模型钠通道 Na V Ab 和人类 Na V 1.7的毒素结合位点,这是一个有吸引力的疼痛目标。这种突变嵌合体使我们能够确定被狼蛛毒素功能性抑制的通道的冷冻电子显微镜(cryo-EM)结构。我们的结构揭示了关键赖氨酸残基之间的高亲和力静息态特异性毒素通道相互作用,该赖氨酸残基充当“毒刺”并穿透电压传感器的 S3-S4 连接体中的三个羧基。通过揭示这种高亲和力结合模式,我们的研究建立了虎纹捕鸟蛛毒素-IV的高分辨率通道对接和静息态锁定机制,并为开发未来静息态靶向镇痛药物提供指导。


























 京公网安备 11010802027423号
京公网安备 11010802027423号