Journal of Inorganic Biochemistry ( IF 3.9 ) Pub Date : 2020-11-23 , DOI: 10.1016/j.jinorgbio.2020.111316 João Paulo L Franco Cairo 1 , David Cannella 2 , Leandro C Oliveira 3 , Thiago A Gonçalves 4 , Marcelo V Rubio 5 , Cesar R F Terrasan 5 , Robson Tramontina 6 , Luciana S Mofatto 7 , Marcelo F Carazzolle 7 , Wanius Garcia 8 , Claus Felby 9 , André Damasio 10 , Paul H Walton 11 , Fabio Squina 6
|
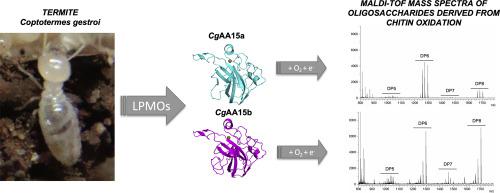
Lytic polysaccharide monooxygenases (LPMOs) are copper-dependent enzymes which catalyze the oxidative cleavage of polysaccharides. LPMOs belonging to family 15 in the Auxiliary Activity (AA) class from the Carbohydrate-Active Enzyme database are found widespread across the Tree of Life, including viruses, algae, oomycetes and animals. Recently, two AA15s from the firebrat Thermobia domestica were reported to have oxidative activity, one towards cellulose or chitin and the other towards chitin, signalling that AA15 LPMOs from insects potentially have different biochemical functions. Herein, we report the identification and characterization of two family AA15 members from the lower termite Coptotermes gestroi. Addition of Cu(II) to CgAA15a or CgAA15b had a thermostabilizing effect on both. Using ascorbate and O2 as co-substrates, CgAA15a and CgAA15b were able to oxidize chitin, but showed no activity on celluloses, xylan, xyloglucan and starch. Structural models indicate that the LPMOs from C. gestroi (CgAA15a/CgAA15b) have a similar fold but exhibit key differences in the catalytic site residues when compared to the cellulose/chitin-active LPMO from T. domestica (TdAA15a), especially the presence of a non-coordinating phenylalanine nearby the Cu ion in CgAA15a/b, which appears as a tyrosine in the active site of TdAA15a. Despite the overall similarity in protein folds, however, mutation of the active site phenylalanine in CgAA15a to a tyrosine did not expanded the enzymatic specificity from chitin to cellulose. Our data show that CgAA15a/b enzymes are likely not involved in lignocellulose digestion but might play a role in termite developmental processes as well as on chitin and nitrogen metabolisms.
中文翻译:

来自白蚁 Coptotermes gestroi 的 AA15 裂解多糖单加氧酶的作用
裂解多糖单加氧酶 (LPMO) 是铜依赖性酶,可催化多糖的氧化裂解。来自碳水化合物活性酶数据库的辅助活性 (AA) 类中的 15 族 LPMO 广泛存在于生命之树中,包括病毒、藻类、卵菌和动物。最近,从小灶衣鱼2个AA15s Thermobia家蝇被报道具有氧化活性,一个朝向纤维素或壳多糖,另一个朝向壳多糖,信令从昆虫AA15 LPMOs潜在地具有不同的生化功能。在此,我们报告了来自下白蚁Coptotermes gestroi的两个 AA15 家族成员的鉴定和表征。将 Cu(II) 添加到Cg AA15a 或CgAA15b 对两者都有热稳定作用。使用抗坏血酸和 O 2作为共底物,Cg AA15a 和Cg AA15b 能够氧化几丁质,但对纤维素、木聚糖、木葡聚糖和淀粉没有活性。结构模型指示来自LPMOs C. gestroi(CG AA15a / CG AA15b)具有相似的折叠但比起从纤维素/几丁质活性LPMO时表现出的催化位点残基主要差异T.家蝇(Td的AA15a),特别是在Cg AA15a/b 中Cu 离子附近存在非配位苯丙氨酸,它在Td的活性位点中表现为酪氨酸AA15a。然而,尽管蛋白质折叠总体相似,但将Cg AA15a 中活性位点苯丙氨酸突变为酪氨酸并没有将酶特异性从几丁质扩展到纤维素。我们的数据显示Cg AA15a/b 酶可能不参与木质纤维素消化,但可能在白蚁发育过程以及几丁质和氮代谢中发挥作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号