Biomaterials ( IF 14.0 ) Pub Date : 2020-11-23 , DOI: 10.1016/j.biomaterials.2020.120546 Wenxi Zhou , Yu Zhou , Xinli Chen , Tingting Ning , Hongyi Chen , Qin Guo , Yiwen Zhang , Peixin Liu , Yujie Zhang , Chao Li , Yongchao Chu , Tao Sun , Chen Jiang
|
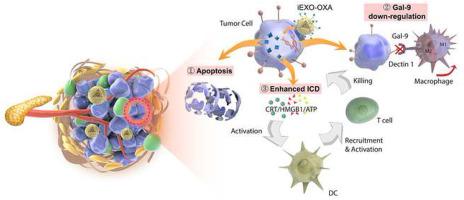
Immunotherapy has gained increasing focus in treating pancreatic ductal adenocarcinoma (PDAC), since conventional therapies like chemotherapy could not provide satisfactory improvement in overall survival outcome of PDAC patients. However, it is still not the game changing solution due to the unique tumor microenvironment and low cancer immunogenicity of PDAC. Thus, inducing more intratumoral effector immune cells as well as reversing immunosuppression is the core of PDAC treatment. Herein, we demonstrate an exosome-based dual delivery biosystem for enhancing PDAC immunotherapy as well as reversing tumor immunosuppression of M2-like tumor associated macrophages (M2-TAMs) upon disruption of galectin-9/dectin 1 axis. The deliver system is constructed from bone marrow mesenchymal stem cell (BM-MSC) exosomes, electroporation-loaded galectin-9 siRNA, and surficially modified with oxaliplatin (OXA) prodrug as an immunogenic cell death (ICD)-trigger. The use of biomaterials, BM-MSC exosomes, can significantly improve tumor targeting efficacy, thus increasing drug accumulation in the tumor site. The combined therapy (iEXO-OXA) elicits anti-tumor immunity through tumor-suppressive macrophage polarization, cytotoxic T lymphocytes recruitment and Tregs downregulation, and achieves significant therapeutic efficacy in cancer treatment.
中文翻译:

靶向胰腺癌的外泌体,用于增强免疫疗法和重编程肿瘤微环境
免疫疗法已成为治疗胰腺导管腺癌(PDAC)的焦点,因为常规疗法(如化学疗法)无法使PDAC患者的总体生存期得到令人满意的改善。然而,由于PDAC独特的肿瘤微环境和低的癌症免疫原性,它仍然不是改变游戏规则的解决方案。因此,诱导更多的肿瘤内效应免疫细胞以及逆转免疫抑制是PDAC治疗的核心。在这里,我们证明了基于外泌体的双重递送生物系统,用于增强PDAC免疫疗法以及在半乳糖凝集素9 / dectin 1轴破坏后逆转M2样肿瘤相关巨噬细胞(M2-TAMs)的肿瘤免疫抑制。该递送系统由骨髓间充质干细胞(BM-MSC)外泌体,电穿孔加载的半乳凝素9 siRNA,并使用奥沙利铂(OXA)前药进行表面修饰,以触发免疫原性细胞死亡(ICD)。BM-MSC外泌体等生物材料的使用可以显着提高肿瘤靶向疗效,从而增加肿瘤部位的药物蓄积。联合疗法(iEXO-OXA)通过抑制肿瘤的巨噬细胞极化,细胞毒性T淋巴细胞募集和Treg下调引发抗肿瘤免疫,并在癌症治疗中取得显着的治疗效果。


























 京公网安备 11010802027423号
京公网安备 11010802027423号