当前位置:
X-MOL 学术
›
FEBS Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The high‐resolution X‐ray structure of vinca domain inhibitors of microtubules provides a rational approach for drug design
FEBS Letters ( IF 3.5 ) Pub Date : 2021-01-01 , DOI: 10.1002/1873-3468.14003 Wu Chengyong 1 , Xian Jinghong 2 , Wang Yanyan 3 , Xiao Qing-Jie 1 , Ma Lingling 4 , Li Yuyan 1 , Chen Hai 4 , Lei Qian 4 , Zhang Quan 5 , Sun Bo 6 , Wang Yuxi 1
FEBS Letters ( IF 3.5 ) Pub Date : 2021-01-01 , DOI: 10.1002/1873-3468.14003 Wu Chengyong 1 , Xian Jinghong 2 , Wang Yanyan 3 , Xiao Qing-Jie 1 , Ma Lingling 4 , Li Yuyan 1 , Chen Hai 4 , Lei Qian 4 , Zhang Quan 5 , Sun Bo 6 , Wang Yuxi 1
Affiliation
|
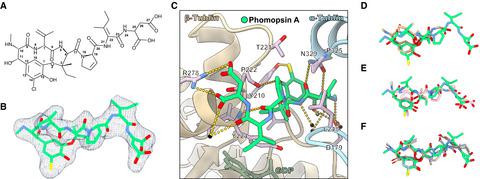
Tubulin vinca‐domain ligands can inhibit microtubule polymerization, causing cell death in mitosis, and their potential against multiple cancer types has been demonstrated. However, due to drug resistance and toxicities, development of novel vinca‐domain ligands is still needed. In this study, we determined the high‐resolution crystal structures of vinorelbine, YXD, and Phomopsin A in complex with tubulin at 2.5 Å. Additionally, we recapitulated all previously published high‐resolution crystal structures of the vinca binding site to reveal critical residues and the molecular mechanism of vinca‐domain ligands interacting with tubulin. Furthermore, we designed putatively novel triazolopyrimidine derivatives by introducing secondary amine groups to establish salt‐bridge and H‐bond interactions with Asp179β1 and Asn329α2. Our studies provided the structural basis for designing novel tubulin vinca‐domain ligands.
中文翻译:

微管长春花域抑制剂的高分辨率 X 射线结构为药物设计提供了合理的方法
微管蛋白长春花结构域配体可以抑制微管聚合,导致有丝分裂中的细胞死亡,并且已证明它们具有对抗多种癌症类型的潜力。然而,由于耐药性和毒性,仍然需要开发新的长春花结构域配体。在这项研究中,我们确定了长春瑞滨、YXD 和 Phomopsin A 与 2.5 Å 微管蛋白复合物的高分辨率晶体结构。此外,我们概括了所有先前发表的长春花结合位点的高分辨率晶体结构,以揭示长春花域配体与微管蛋白相互作用的关键残基和分子机制。此外,我们通过引入仲胺基团来与 Asp179β1 和 Asn329α2 建立盐桥和 H 键相互作用,设计了推定的新型三唑并嘧啶衍生物。
更新日期:2021-01-01
中文翻译:

微管长春花域抑制剂的高分辨率 X 射线结构为药物设计提供了合理的方法
微管蛋白长春花结构域配体可以抑制微管聚合,导致有丝分裂中的细胞死亡,并且已证明它们具有对抗多种癌症类型的潜力。然而,由于耐药性和毒性,仍然需要开发新的长春花结构域配体。在这项研究中,我们确定了长春瑞滨、YXD 和 Phomopsin A 与 2.5 Å 微管蛋白复合物的高分辨率晶体结构。此外,我们概括了所有先前发表的长春花结合位点的高分辨率晶体结构,以揭示长春花域配体与微管蛋白相互作用的关键残基和分子机制。此外,我们通过引入仲胺基团来与 Asp179β1 和 Asn329α2 建立盐桥和 H 键相互作用,设计了推定的新型三唑并嘧啶衍生物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号