当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Novel Pyridine‐Based Hydroxamates and 2′‐Aminoanilides as Histone Deacetylase Inhibitors: Biochemical Profile and Anticancer Activity
ChemMedChem ( IF 3.4 ) Pub Date : 2020-11-21 , DOI: 10.1002/cmdc.202000854 Clemens Zwergel 1 , Elisabetta Di Bello 1 , Rossella Fioravanti 1 , Mariarosaria Conte 2 , Angela Nebbioso 2 , Roberta Mazzone 1 , Gerald Brosch 3 , Ciro Mercurio 4 , Mario Varasi 4 , Lucia Altucci 2 , Sergio Valente 1 , Antonello Mai 1
ChemMedChem ( IF 3.4 ) Pub Date : 2020-11-21 , DOI: 10.1002/cmdc.202000854 Clemens Zwergel 1 , Elisabetta Di Bello 1 , Rossella Fioravanti 1 , Mariarosaria Conte 2 , Angela Nebbioso 2 , Roberta Mazzone 1 , Gerald Brosch 3 , Ciro Mercurio 4 , Mario Varasi 4 , Lucia Altucci 2 , Sergio Valente 1 , Antonello Mai 1
Affiliation
|
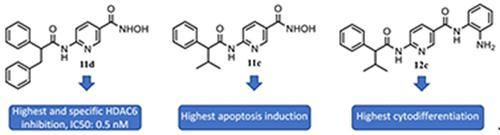
Starting from the N‐hydroxy‐3‐(4‐(2‐phenylbutanoyl)amino)phenyl)acrylamide (5 b) previously described by us as a HDAC inhibitor, we prepared four aza‐analogues, 6–8, 9 b, as regioisomers containing the pyridine nucleus. Preliminary screening against mHDAC1 highlighted the N‐hydroxy‐5‐(2‐(2‐phenylbutanoyl)amino)pyridyl)acrylamide (9 b) as the most potent inhibitor. Thus, we further developed both pyridylacrylic‐ and nicotinic‐based hydroxamates (9 a, 9 c–f, and 11 a–f) and 2′‐aminoanilides (10 a–f and 12 a–f), related to 9 b, to be tested against HDACs. Among them, the nicotinic hydroxamate 11 d displayed sub‐nanomolar potency (IC50: 0.5 nM) and selectivity up to 34 000 times that of HDAC4 and from 100 to 1300 times that of all the other tested HDAC isoforms. The 2′‐aminoanilides were class I‐selective HDAC inhibitors, generally more potent against HDAC3, with the nicotinic anilide 12 d being the most effective (IC50HDAC3=0.113 μM). When tested in U937 leukemia cells, the hydroxamates 9 e, 11 c, and 11 d blocked over 80 % of cells in G2/M phase, whereas the anilides did not alter cell‐cycle progress. In the same cell line, the hydroxamate 11 c and the anilide 10 b induced about 30 % apoptosis, and the anilide 12 c displayed about 40 % cytodifferentiation. Finally, the most potent compounds in leukemia cells 9 b, 11 c, 10 b, 10 e, and 12 c were also tested in K562, HCT116, and A549 cancer cells, displaying antiproliferative IC50 values at single‐digit to sub‐micromolar level.
中文翻译:

新型吡啶基异羟肟酸盐和 2'-氨基苯胺作为组蛋白脱乙酰酶抑制剂:生化特征和抗癌活性
从起始Ñ -羟基-3-(4-(2-苯基丁酰基)氨基)苯基)丙烯酰胺(图5b之前由我们描述为HDAC抑制剂),我们制备了4氮杂类似物,6 - 8,9b中,如含有吡啶核的区域异构体。针对 mHDAC1 的初步筛选突出显示了N-羟基-5-(2-(2-苯基丁酰基)氨基)吡啶基)丙烯酰胺 ( 9b ) 作为最有效的抑制剂。因此,我们进一步发展既pyridylacrylic-和烟碱基异羟肟酸盐(9,9c的- ˚F,和11 - ˚F)和2'-aminoanilides(10 -f和12 a – f ),与9 b相关,针对 HDAC 进行测试。其中,烟碱异羟肟酸酯11 d显示出亚纳摩尔的效力(IC 50:0.5 nM)和选择性高达 HDAC4 的 34 000 倍和所有其他测试的 HDAC 同种型的 100 至 1300 倍。2'-氨基苯胺是 I 类选择性 HDAC 抑制剂,通常对 HDAC3 更有效,其中烟碱苯胺12 天最有效(IC 50 HDAC3 =0.113 μM)。当在U937白血病细胞进行试验时,异羟肟酸盐9e的,11c中,和11 d在 G2/M 期阻断了超过 80% 的细胞,而苯胺并没有改变细胞周期进程。在相同的细胞系,氧肟酸盐11c中和N-酰苯胺10b中诱导约30周%的凋亡,而苯胺12c中显示约40%的细胞分化。最后,在白血病细胞中最有效的化合物9b中,11c中,10b中,10e中,和12c中的K562,HCT116,和A549癌细胞也进行了测试,并显示抗增殖IC 50到亚微摩尔在单数位值等级。
更新日期:2020-11-21
中文翻译:

新型吡啶基异羟肟酸盐和 2'-氨基苯胺作为组蛋白脱乙酰酶抑制剂:生化特征和抗癌活性
从起始Ñ -羟基-3-(4-(2-苯基丁酰基)氨基)苯基)丙烯酰胺(图5b之前由我们描述为HDAC抑制剂),我们制备了4氮杂类似物,6 - 8,9b中,如含有吡啶核的区域异构体。针对 mHDAC1 的初步筛选突出显示了N-羟基-5-(2-(2-苯基丁酰基)氨基)吡啶基)丙烯酰胺 ( 9b ) 作为最有效的抑制剂。因此,我们进一步发展既pyridylacrylic-和烟碱基异羟肟酸盐(9,9c的- ˚F,和11 - ˚F)和2'-aminoanilides(10 -f和12 a – f ),与9 b相关,针对 HDAC 进行测试。其中,烟碱异羟肟酸酯11 d显示出亚纳摩尔的效力(IC 50:0.5 nM)和选择性高达 HDAC4 的 34 000 倍和所有其他测试的 HDAC 同种型的 100 至 1300 倍。2'-氨基苯胺是 I 类选择性 HDAC 抑制剂,通常对 HDAC3 更有效,其中烟碱苯胺12 天最有效(IC 50 HDAC3 =0.113 μM)。当在U937白血病细胞进行试验时,异羟肟酸盐9e的,11c中,和11 d在 G2/M 期阻断了超过 80% 的细胞,而苯胺并没有改变细胞周期进程。在相同的细胞系,氧肟酸盐11c中和N-酰苯胺10b中诱导约30周%的凋亡,而苯胺12c中显示约40%的细胞分化。最后,在白血病细胞中最有效的化合物9b中,11c中,10b中,10e中,和12c中的K562,HCT116,和A549癌细胞也进行了测试,并显示抗增殖IC 50到亚微摩尔在单数位值等级。


























 京公网安备 11010802027423号
京公网安备 11010802027423号