Microporous and Mesoporous Materials ( IF 5.2 ) Pub Date : 2020-11-22 , DOI: 10.1016/j.micromeso.2020.110773 M. Mahima Kumar , K.A. Irshad , Hrudananda Jena
|
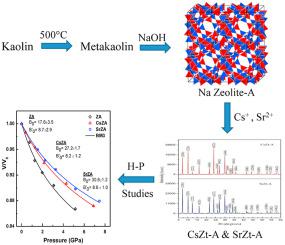
Zeolite-A was synthesized from kaolin activated at 500 °C at relatively lower temperature than the studies reported in the literature.The metakaolin formed at 500 °C was used to synthesise zeolite-A by treating with 1–5 M NaOH solutions at 80–100 °C for 3–24 h under hydrothermal reaction condition. The zeolite-A was characterized by XRD, FT-IR and SEM. The percentage of crystallinity and crystallite sizes of the zeolite-A were determined by using Rietveld analysis; the crystal system of zeolite-A was found to be cubic with space group ( ). The ion-exchange property of zeolite-A for Cs+ and Sr+2 was explored at ambient temperature (25 °C). The adsorption efficiency of zeolite-A is higher for Sr+2 than that of Cs+; and adsorption capacities for Cs+ and Sr2+ ions were found to be 91.51% and 99.16% respectively. Strontium substituted zeolite has higher crystallinity and lattice strain than that of caesium substituted zeolite-A. Structural stability investigations were carried out on pristine zeolite-A and its Cs+ and Sr2+ substituted analogues by using high-pressure (0.1 MPa-11.4 GPa) X-ray diffractometry technique for the first time. The pristine zeolite-A shows comparatively lower bulk modulus than that of caesium and strontium loaded zeolite. It is also seen that the compressibility is least for the strontium loaded zeolite-A (SrZtA) followed by caesium loaded zeolite-A (CsZtA) and pristine zeolite-A (ZtA) (i.e compressibility of SrZtA < compressibility of CsZtA < compressibility of ZtA).
中文翻译:

使用高岭土合成的Zeolite-A从模拟放射性废物溶液中去除Cs +和Sr 2+离子及其在高压下的结构稳定性
沸石A是由在500°C下活化的高岭土合成的,合成温度低于文献报道的温度。在500°C下形成的偏高岭土通过在80–80℃下用1–5 M NaOH溶液处理来合成沸石在水热反应条件下100°C持续3–24 h。通过XRD,FT-IR和SEM对沸石-A进行了表征。使用Rietveld分析确定沸石-A的结晶度百分数和晶粒尺寸。发现A型分子筛的晶体系统为立方晶且空间群为()。在环境温度(25°C)下研究了沸石-A对Cs +和Sr +2的离子交换性能。沸石-A对Sr +2的吸附效率高于对Cs +的吸附效率;Cs +和Sr 2+离子的吸附容量分别为91.51%和99.16%。锶取代的沸石具有比铯取代的沸石-A更高的结晶度和晶格应变。对原始沸石-A及其Cs +和Sr 2+进行结构稳定性研究首次使用高压(0.1 MPa-11.4 GPa)X射线衍射技术替代了类似物。原始沸石-A的体积模量比铯和锶负载的沸石低。还可以看出,锶负载沸石-A(SrZtA)紧随其后,铯负载沸石-A(CsZtA)和原始沸石-A(ZtA)的可压缩性最低(即,SrZtA的压缩性<CsZtA的压缩性<ZtA的压缩性)。


























 京公网安备 11010802027423号
京公网安备 11010802027423号