当前位置:
X-MOL 学术
›
Hydrometallurgy
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A process for beneficiation of low-grade manganese ore and synchronous preparation of calcium sulfate whiskers during hydrochloric acid regeneration
Hydrometallurgy ( IF 4.7 ) Pub Date : 2021-02-01 , DOI: 10.1016/j.hydromet.2020.105533 Mengen Lei , Baozhong Ma , Dongya Lv , Chengyan Wang , Edouard Asselin , Yongqiang Chen
Hydrometallurgy ( IF 4.7 ) Pub Date : 2021-02-01 , DOI: 10.1016/j.hydromet.2020.105533 Mengen Lei , Baozhong Ma , Dongya Lv , Chengyan Wang , Edouard Asselin , Yongqiang Chen
|
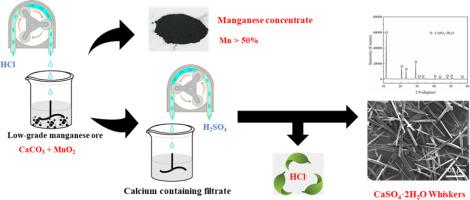
Abstract In order to economically utilize low-grade manganese ores in Indonesia, a process is proposed to produce high-grade manganese concentrate and prepare calcium sulfate whiskers. Low-grade manganese ore is beneficiated with hydrochloric acid as a leaching agent to obtain a manganese concentrate. Hydrochloric acid can then be regenerated using cheaper sulfuric acid via contact with the leach liquor (a CaCl2 solution), whereas calcium sulfate whiskers can also be precipitated during this regeneration. Herein, the effects of pH and reaction time on the leaching of manganese ore were evaluated, and the effects of reaction temperature, stirring speed, precipitation time, calcium ion concentration and sulfuric acid concentrate on the morphology of the calcium sulfate whiskers were experimentally investigated. The products were analyzed by means of scanning electron microscopy (SEM), thermogravimetric-differential scanning calorimetry (TG-DSC), and X-ray diffraction (XRD). The results show that control pH to 3 and leaching duration is 1.5 h, the grade of manganese ore increases from 16.4% to more than 51.6%. The CaSO4·2H2O whiskers prepared have good crystallinity, uniform morphology, high aspect ratio and good reproducibility under the optimum conditions: 50 °C, stirring speed of 250 r·min−1, precipitation time of 1 h, calcium ion initial concentration of 30 g·L−1, and sulfuric acid concentration is 30.8%. In addition, CaSO4·0.5H2O whiskers with high aspect ratio can also be prepared under atmospheric pressure. The phase transformation of CaSO4·2H2O is also studied. In low concentration hydrochloric acid (1 mol·L−1), the main product is CaSO4·2H2O. In high concentration hydrochloric acid (2.5 mol·L−1), when the reaction time is less than 2 h, CaSO4·0.5H2O occurs only when the temperature reaches 102 °C. When the reaction time is not less than 8 h, and the temperature is higher than 95 °C, CaSO4 is produced. A manganese concentrate (Mn, more than 51%) can be obtained via the regenerated hydrochloric acid leaching.
中文翻译:

盐酸再生低品位锰矿选矿与硫酸钙晶须同步制备工艺
摘要 为了经济利用印尼低品位锰矿,提出了一种生产高品位锰精矿和制备硫酸钙晶须的工艺。以盐酸为浸出剂对低品位锰矿进行选矿,得到锰精矿。然后可以使用更便宜的硫酸通过与浸出液(CaCl2 溶液)接触来再生盐酸,而硫酸钙晶须也可以在再生过程中沉淀。在此,评价了pH和反应时间对锰矿浸出的影响,并通过实验研究了反应温度、搅拌速度、沉淀时间、钙离子浓度和硫酸精矿对硫酸钙晶须形貌的影响。通过扫描电子显微镜(SEM)、热重-差示扫描量热法(TG-DSC)和X射线衍射(XRD)对产物进行分析。结果表明,控制pH为3,浸出时间为1.5 h,锰矿品位由16.4%提高到51.6%以上。制备的CaSO4·2H2O晶须在50℃、搅拌速度250 r·min−1、沉淀时间1 h、钙离子初始浓度30 g·L-1,硫酸浓度为30.8%。此外,在常压下也可以制备高纵横比的CaSO4·0.5H2O晶须。还研究了CaSO4·2H2O的相变。在低浓度盐酸(1 mol·L-1)中,主要产物为CaSO4·2H2O。在高浓度盐酸(2.5 mol·L-1)中,当反应时间小于2 h时,CaSO4·0.5H2O只有在温度达到102℃时才会发生。当反应时间不少于8h,温度高于95℃时,生成CaSO4。再生盐酸浸出可得到锰精矿(Mn,大于51%)。
更新日期:2021-02-01
中文翻译:

盐酸再生低品位锰矿选矿与硫酸钙晶须同步制备工艺
摘要 为了经济利用印尼低品位锰矿,提出了一种生产高品位锰精矿和制备硫酸钙晶须的工艺。以盐酸为浸出剂对低品位锰矿进行选矿,得到锰精矿。然后可以使用更便宜的硫酸通过与浸出液(CaCl2 溶液)接触来再生盐酸,而硫酸钙晶须也可以在再生过程中沉淀。在此,评价了pH和反应时间对锰矿浸出的影响,并通过实验研究了反应温度、搅拌速度、沉淀时间、钙离子浓度和硫酸精矿对硫酸钙晶须形貌的影响。通过扫描电子显微镜(SEM)、热重-差示扫描量热法(TG-DSC)和X射线衍射(XRD)对产物进行分析。结果表明,控制pH为3,浸出时间为1.5 h,锰矿品位由16.4%提高到51.6%以上。制备的CaSO4·2H2O晶须在50℃、搅拌速度250 r·min−1、沉淀时间1 h、钙离子初始浓度30 g·L-1,硫酸浓度为30.8%。此外,在常压下也可以制备高纵横比的CaSO4·0.5H2O晶须。还研究了CaSO4·2H2O的相变。在低浓度盐酸(1 mol·L-1)中,主要产物为CaSO4·2H2O。在高浓度盐酸(2.5 mol·L-1)中,当反应时间小于2 h时,CaSO4·0.5H2O只有在温度达到102℃时才会发生。当反应时间不少于8h,温度高于95℃时,生成CaSO4。再生盐酸浸出可得到锰精矿(Mn,大于51%)。



























 京公网安备 11010802027423号
京公网安备 11010802027423号